J Bone Metab.
2018 Feb;25(1):43-51. 10.11005/jbm.2018.25.1.43.
The Effect of Antidepressants on Mesenchymal Stem Cell Differentiation
- Affiliations
-
- 1Sydney Medical School Nepean, The University of Sydney, Penrith, Australia. gustavo.duque@unimelb.edu.au
- 2Facultad de Ciencias Básicas y Biomédicas, Universidad Simón BolÃvar, Barranquilla, Colombia.
- 3Australian Institute for Musculoskeletal Science (AIMSS), The University of Melbourne and Western Health, Melbourne, Australia.
- 4Department of Medicine-Western Health, Melbourne Medical School, The University of Melbourne, Melbourne, Australia.
- KMID: 2406238
- DOI: http://doi.org/10.11005/jbm.2018.25.1.43
Abstract
- BACKGROUND
Use of antidepressant medications has been linked to detrimental impacts on bone mineral density and osteoporosis; however, the cellular basis behind these observations remains poorly understood. The effect does not appear to be homogeneous across the whole class of drugs and may be linked to affinity for the serotonin transporter system. In this study, we hypothesized that antidepressants have a class- and dose-dependent effect on mesenchymal stem cell (MSC) differentiation, which may affect bone metabolism.
METHODS
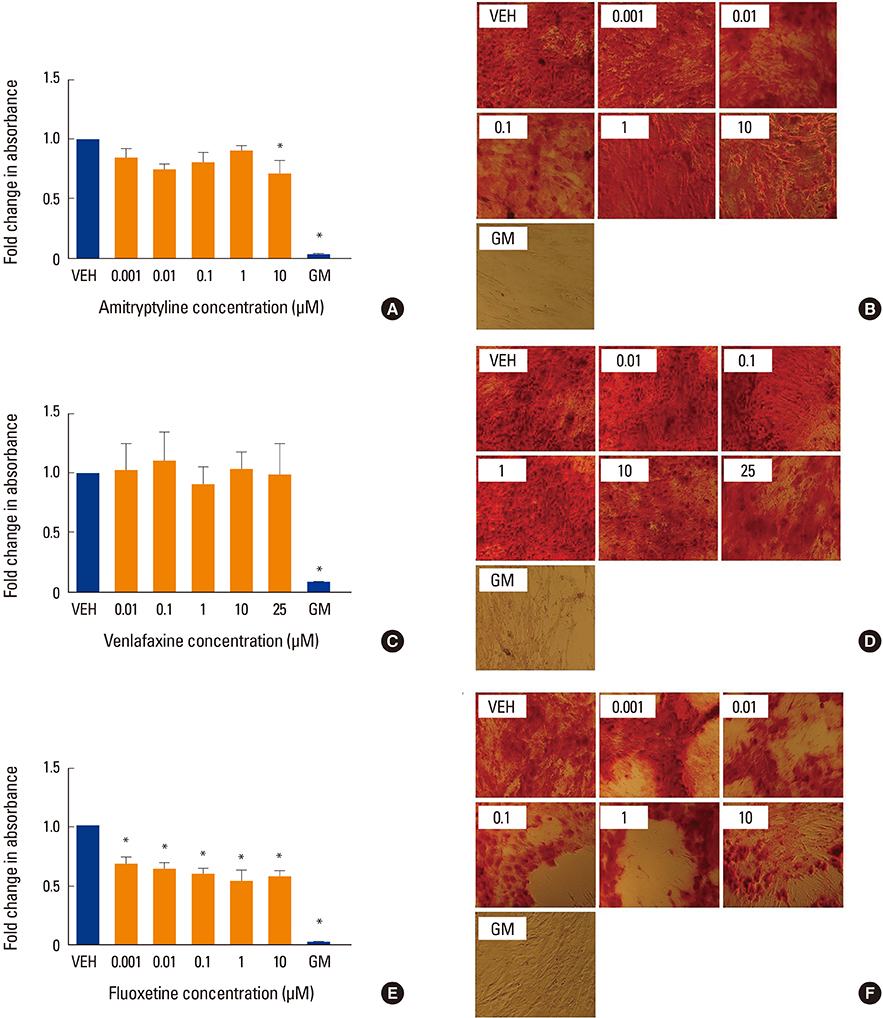
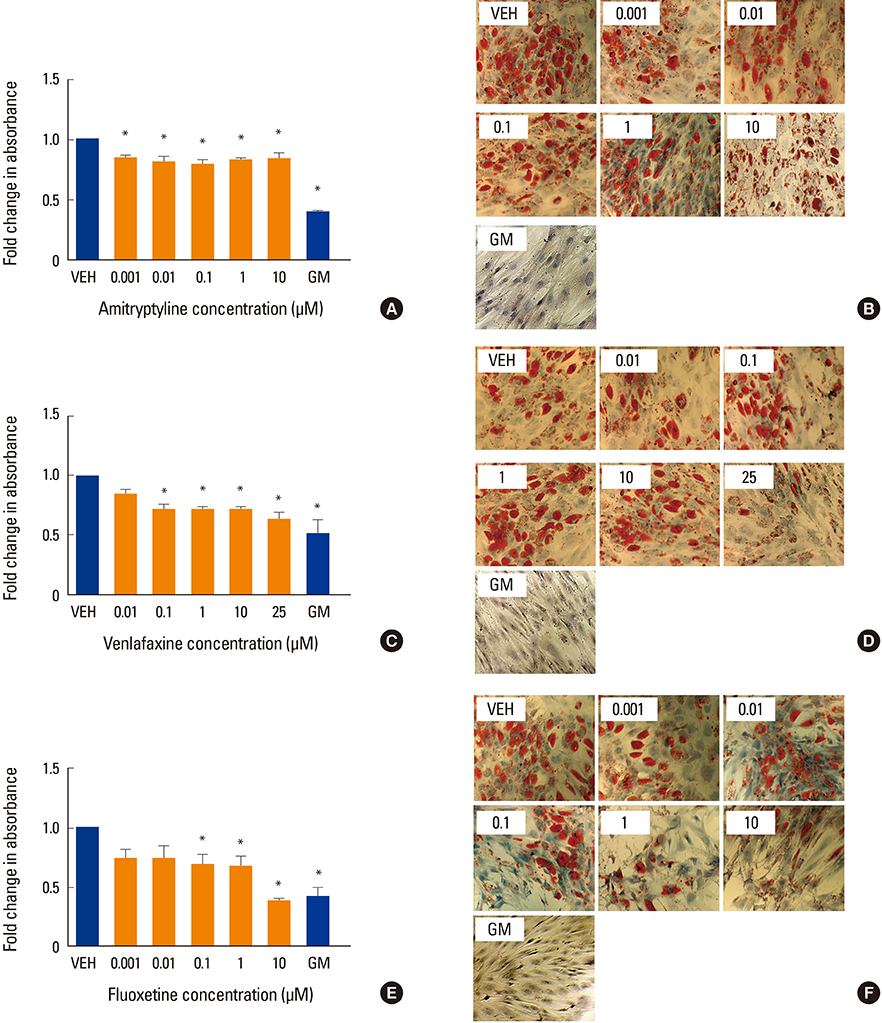
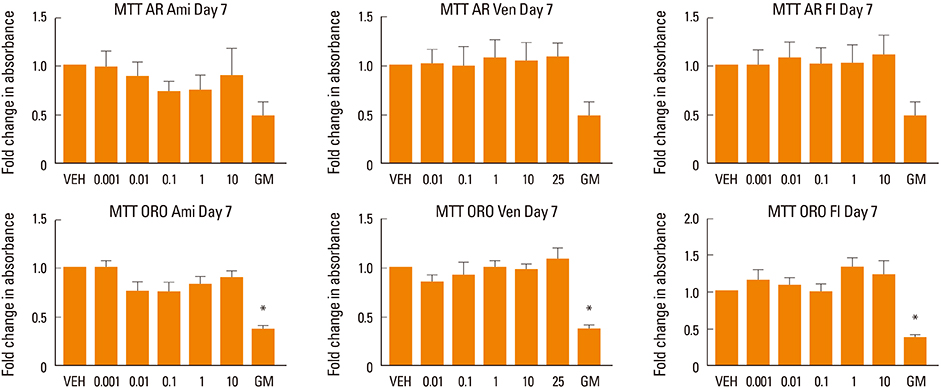
Human MSCs (hMSCs) were committed to differentiate when either adipogenic or osteogenic media was added, supplemented with five increasing concentrations of amitriptyline (0.001-10 µM), venlafaxine (0.01-25 µM), or fluoxetine (0.001-10 µM). Alizarin red staining (mineralization), alkaline phosphatase (osteoblastogenesis), and oil red O (adipogenesis) assays were performed at timed intervals. In addition, cell viability was assessed using a MTT.
RESULTS
We found that fluoxetine had a significant inhibitory effect on mineralization. Furthermore, adipogenic differentiation of hMSC was affected by the addition of amitriptyline, venlafaxine, and fluoxetine to the media. Finally, none of the tested medications significantly affected cell survival.
CONCLUSIONS
This study showed a divergent effect of three antidepressants on hMSC differentiation, which appears to be independent of class and dose. As fluoxetine and amitriptyline, but not venlafaxine, affected both osteoblastogenesis and adipogenesis, this inhibitory effect could be associated to the high affinity of fluoxetine to the serotonin transporter system.
MeSH Terms
-
Adipogenesis
Alkaline Phosphatase
Amitriptyline
Antidepressive Agents*
Bone Density
Cell Survival
Fluoxetine
Humans
Mesenchymal Stromal Cells*
Metabolism
Miners
Osteoblasts
Osteoporosis
Serotonin Plasma Membrane Transport Proteins
Venlafaxine Hydrochloride
Alkaline Phosphatase
Amitriptyline
Antidepressive Agents
Fluoxetine
Serotonin Plasma Membrane Transport Proteins
Venlafaxine Hydrochloride
Figure
Reference
-
1. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016; 374:254–262.2. Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic differentiation in healthy and pathological conditions. Int J Mol Sci. 2016; 18:41.
Article3. Verma S, Rajaratnam JH, Denton J, et al. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002; 55:693–698.
Article4. Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004; 23:183–188.
Article5. Bermeo S, Gunaratnam K, Duque G. Fat and bone interactions. Curr Osteoporos Rep. 2014; 12:235–242.
Article6. James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo). 2013; 2013:684736.
Article7. Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010; 21:1045–1056.
Article8. Jacobs SA, Roobrouck VD, Verfaillie CM, et al. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013; 91:32–39.
Article9. Zhang Y, Khan D, Delling J, et al. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal. 2012; 2012:793823.
Article10. Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012; 81:715–736.
Article11. Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008; 9:1393–1402.
Article12. Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008; 172:625–646.
Article13. Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009; 61:761–777.
Article14. Geldenhuys WJ, Van der Schyf CJ. The serotonin 5-HT6 receptor: a viable drug target for treating cognitive deficits in Alzheimer's disease. Expert Rev Neurother. 2009; 9:1073–1085.
Article15. Abrams JK, Johnson PL, Hollis JH, et al. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004; 1018:46–57.
Article16. Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003; 26:331–343.
Article17. Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus-from silver stainings to a role in depression. Brain Res Rev. 2007; 55:329–342.
Article18. Gustafsson BI, Thommesen L, Stunes AK, et al. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006; 98:139–151.
Article19. Dimitri P, Rosen C. The central nervous system and bone metabolism: an evolving story. Calcif Tissue Int. 2017; 100:476–485.
Article20. Oh CM, Park S, Kim H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J. 2016; 40:89–98.
Article21. Namkung J, Kim H, Park S. Peripheral serotonin: a new player in systemic energy homeostasis. Mol Cells. 2015; 38:1023–1028.
Article22. Karsenty G, Yadav VK. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med. 2011; 62:323–331.
Article23. Tatsumi M, Groshan K, Blakely RD, et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997; 340:249–258.
Article24. Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28:435–451.
Article25. Holtzheimer PE 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx. 2006; 3:42–56.
Article26. Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010; 31:60–65.
Article27. Magni LR, Purgato M, Gastaldon C, et al. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev. 2013; Cd004185.
Article28. Owens MJ, Morgan WN, Plott SJ, et al. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997; 283:1305–1322.29. Li B, Zhang S, Zhang H, et al. Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology (Berl). 2008; 201:443–458.
Article30. Li B, Zhang S, Li M, et al. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT2B-induced, transactivation-mediated ERK1/2 phosphorylation. Psychopharmacology (Berl). 2009; 207:1–12.
Article31. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001; 25:871–880.
Article32. Wong SY, Lau EM, Lynn H, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong). Osteoporos Int. 2005; 16:610–615.
Article33. Silverman SL, Shen W, Minshall ME, et al. Prevalence of depressive symptoms in postmenopausal women with low bone mineral density and/or prevalent vertebral fracture: results from the Multiple Outcomes of Raloxifene Evaluation (MORE) study. J Rheumatol. 2007; 34:140–144.34. Erez HB, Weller A, Vaisman N, et al. The relationship of depression, anxiety and stress with low bone mineral density in post-menopausal women. Arch Osteoporos. 2012; 7:247–255.
Article35. Robbins J, Hirsch C, Whitmer R, et al. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001; 49:732–736.
Article36. Diem SJ, Blackwell TL, Stone KL, et al. Depressive symptoms and rates of bone loss at the hip in older women. J Am Geriatr Soc. 2007; 55:824–831.
Article37. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016; 33:21–25.
Article38. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article39. Vidal C, Li W, Santner-Nanan B, et al. The kynurenine pathway of tryptophan degradation is activated during osteoblastogenesis. Stem Cells. 2015; 33:111–121.
Article40. Pälvimäki EP, Roth BL, Majasuo H, et al. Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor. Psychopharmacology (Berl). 1996; 126:234–240.
Article41. Kruk JS, Vasefi MS, Gondora N, et al. Fluoxetine-induced transactivation of the platelet-derived growth factor type beta receptor reveals a novel heterologous desensitization process. Mol Cell Neurosci. 2015; 65:45–51.
Article42. Nam SS, Lee JC, Kim HJ, et al. Serotonin inhibits osteoblast differentiation and bone regeneration in rats. J Periodontol. 2016; 87:461–469.
Article43. Bradaschia-Correa V, Josephson AM, Mehta D, et al. The selective serotonin reuptake inhibitor fluoxetine directly inhibits osteoblast differentiation and mineralization during fracture healing in mice. J Bone Miner Res. 2017; 32:821–833.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- The Role of microRNAs Involved in Mesenchymal Stem Cell Differentiation
- Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients