J Breast Cancer.
2017 Mar;20(1):20-26. 10.4048/jbc.2017.20.1.20.
Inhibition of Nicotinamide Phosphoribosyltransferase Induces Apoptosis in Estrogen Receptor-Positive MCF-7 Breast Cancer Cells
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
- 2Department of Biochemistry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. Nourbakhsh.m@iums.ac.ir
- KMID: 2379395
- DOI: http://doi.org/10.4048/jbc.2017.20.1.20
Abstract
- PURPOSE
Tumor cells have increased turnover of nicotinamide adenine dinucleotide (NADâº), the main coenzyme in processes including adenosine diphosphate-ribosylation, deacetylation, and calcium mobilization. NAD⺠is predominantly synthesized in human cells via the salvage pathway, with the first component being nicotinamide. Nicotinamide phosphoribosyltransferase (NAMPT) is the key enzyme in this pathway, and its chemical inhibition by FK866 has elicited antitumor effects in several preclinical models of solid and hematologic cancers. However, its efficacy in estrogen receptor (ER)-positive and human epidermal growth factor receptor 2-positive breast cancer cells has not been previously investigated. In this study, we aimed to deplete the NAD⺠content of MCF-7 cells, a model cell line for ER-positive breast cancer, by inhibiting NAMPT in order to evaluate downstream effects on p53 and its acetylation, p21 and Bcl-2-associated X protein (BAX) expression, and finally, apoptosis in MCF-7 breast cancer cells.
METHODS
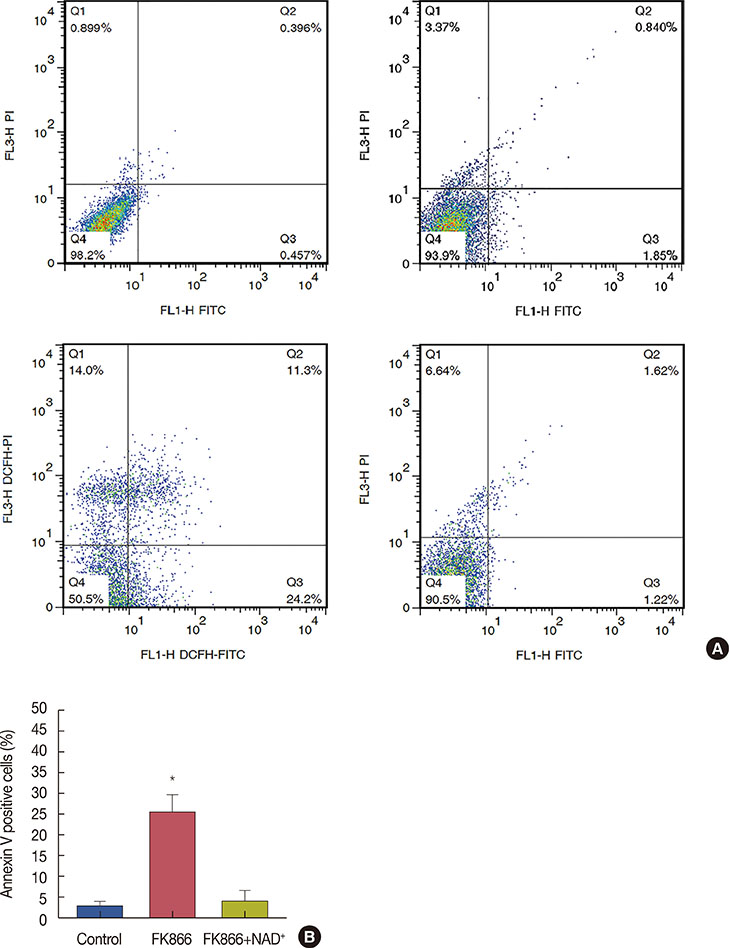
MCF-7 cells were cultured and treated with FK866. NAD⺠levels in cells were determined colorimetrically. Levels of p53 and its acetylated form were determined by Western blotting. Expression of p21 and BAX was determined by real-time polymerase chain reaction. Finally, levels of apoptosis were assessed by flow cytometry using markers for annexin V and propidium iodide.
RESULTS
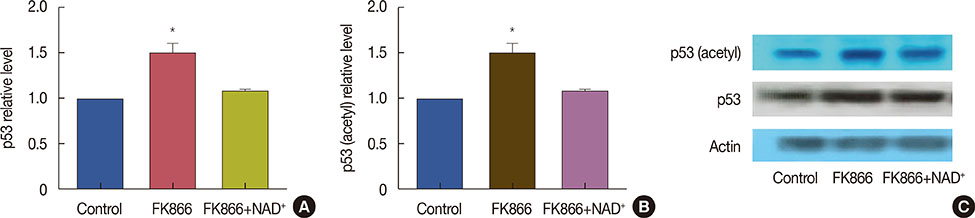
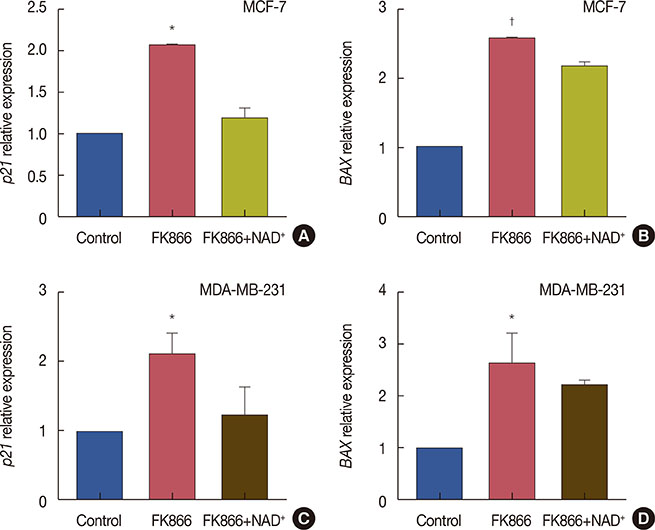
FK866 treatment was able to increase p53 levels and acetylation, upregulate BAX and p21 expression, and induce apoptosis in MCF-7 cells. Addition of exogenous NAD⺠to cells reversed these effects, suggesting that FK866 exerted its effects by depleting NAD⺠levels.
CONCLUSION
Results showed that FK866 could effectively inhibit NAD⺠biosynthesis and induce programmed cell death in MCF-7 cells, suggesting that NAMPT inhibitors may be useful for the treatment of ER-positive breast cancers.
Keyword
MeSH Terms
-
Acetylation
Adenosine
Annexin A5
Apoptosis*
bcl-2-Associated X Protein
Blotting, Western
Breast Neoplasms*
Breast*
Calcium
Cell Death
Cell Line
Estrogens*
Flow Cytometry
Humans
MCF-7 Cells
NAD
Niacinamide*
Nicotinamide Phosphoribosyltransferase*
Propidium
Real-Time Polymerase Chain Reaction
Receptor, Epidermal Growth Factor
Tumor Suppressor Protein p53
Adenosine
Annexin A5
Calcium
Estrogens
NAD
Niacinamide
Nicotinamide Phosphoribosyltransferase
Propidium
Receptor, Epidermal Growth Factor
Tumor Suppressor Protein p53
bcl-2-Associated X Protein
Figure
Reference
-
1. Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome: a key determinant of cancer cell biology. Nat Rev Cancer. 2012; 12:741–752.
Article2. Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol. 2012; 199:205–209.
Article3. Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015; 50:284–297.
Article4. Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015; 11:535–546.
Article5. Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003; 63:7436–7442.6. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010; 107:2037–2042.
Article7. Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007; 130:1095–1107.
Article8. Thakur BK, Dittrich T, Chandra P, Becker A, Kuehnau W, Klusmann JH, et al. Involvement of p53 in the cytotoxic activity of the NAMPT inhibitor FK866 in myeloid leukemic cells. Int J Cancer. 2013; 132:766–774.
Article9. Chini CC, Guerrico AM, Nin V, Camacho-Pereira J, Escande C, Barbosa MT, et al. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res. 2014; 20:120–130.
Article10. Cea M, Soncini D, Fruscione F, Raffaghello L, Garuti A, Emionite L, et al. Synergistic interactions between HDAC and sirtuin inhibitors in human leukemia cells. PLoS One. 2011; 6:e22739.
Article11. Walt G. WHO's world health report 2003. BMJ. 2004; 328:6.
Article12. Bowlby SC, Thomas MJ, D'Agostino RB Jr, Kridel SJ. Nicotinamide phosphoribosyl transferase (Nampt) is required for de novo lipogenesis in tumor cells. PLoS One. 2012; 7:e40195.
Article13. Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009; 20:130–138.
Article14. Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008; 3:e2267.
Article15. Holen K, Saltz LB, Hollywood E, Burk K, Hanauske AR. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest New Drugs. 2008; 26:45–51.
Article16. Bajrami I, Kigozi A, Van Weverwijk A, Brough R, Frankum J, Lord CJ, et al. Synthetic lethality of PARP and NAMPT inhibition in triple-negative breast cancer cells. EMBO Mol Med. 2012; 4:1087–1096.
Article17. Barrios CH, Sampaio C, Vinholes J, Caponero R. What is the role of chemotherapy in estrogen receptor-positive, advanced breast cancer? Ann Oncol. 2009; 20:1157–1162.
Article18. Lønning PE. Poor-prognosis estrogen receptor-positive disease: present and future clinical solutions. Ther Adv Med Oncol. 2012; 4:127–137.
Article19. Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, et al. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009; 284:20408–20417.
Article20. Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008; 133:612–626.
Article21. Yamaguchi H, Woods NT, Piluso LG, Lee HH, Chen J, Bhalla KN, et al. p53 acetylation is crucial for its transcription-independent proapoptotic functions. J Biol Chem. 2009; 284:11171–11183.
Article22. Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001; 107:137–148.
Article23. Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002; 21:6236–6245.
Article24. Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994; 9:1799–1805.25. Thakur BK, Dittrich T, Chandra P, Becker A, Lippka Y, Selvakumar D, et al. Inhibition of NAMPT pathway by FK866 activates the function of p53 in HEK293T cells. Biochem Biophys Res Commun. 2012; 424:371–377.
Article26. Montecucco F, Cea M, Bauer I, Soncini D, Caffa I, Lasigliè D, et al. Nicotinamide phosphoribosyltransferase (NAMPT) inhibitors as therapeutics: rationales, controversies, clinical experience. Curr Drug Targets. 2013; 14:637–643.
Article27. Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009; 284:10334–10342.
Article28. Shukla S, Sharma A, Pandey VK, Raisuddin S, Kakkar P. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharmacol. 2016; 291:70–83.
Article29. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005; 24:7410–7425.
Article30. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011; 30:87.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Leptin as a Potential Target for Estrogen Receptor-Positive Breast Cancer
- Effects of retinoic acid isomers on apoptosis and enzymatic antioxidant system in human breast cancer cells
- Up-regulation of P13K/Akt Signaling by 17 beta-estradiol through Activation of Estrogen Receptor-alpha in Breast Cancer Cells