J Breast Cancer.
2013 Jun;16(2):138-145. 10.4048/jbc.2013.16.2.138.
Leptin as a Potential Target for Estrogen Receptor-Positive Breast Cancer
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. dynoh@snu.ac.kr
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2286392
- DOI: http://doi.org/10.4048/jbc.2013.16.2.138
Abstract
- PURPOSE
Leptin is a potent adipokine that plays a significant role in tumor development and the progression of breast cancer. The aim of this study was to evaluate whether leptin affects the response to tamoxifen treatment in estrogen receptor (ER)-positive breast cancer cells.
METHODS
Leptin, leptin receptor (Ob-R), and activation of signaling pathways were studied by Western immunoblotting. The effects of leptin on tamoxifen-dependent growth inhibition were studied in MCF-7 and T-47D cells.
RESULTS
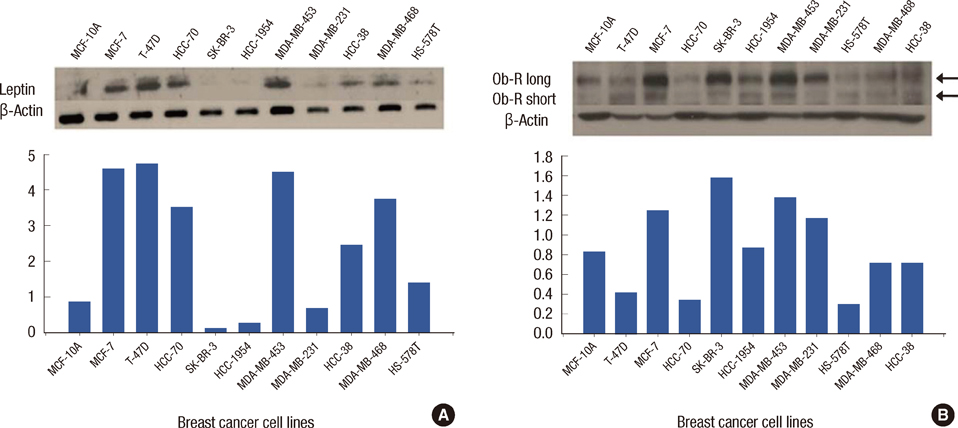
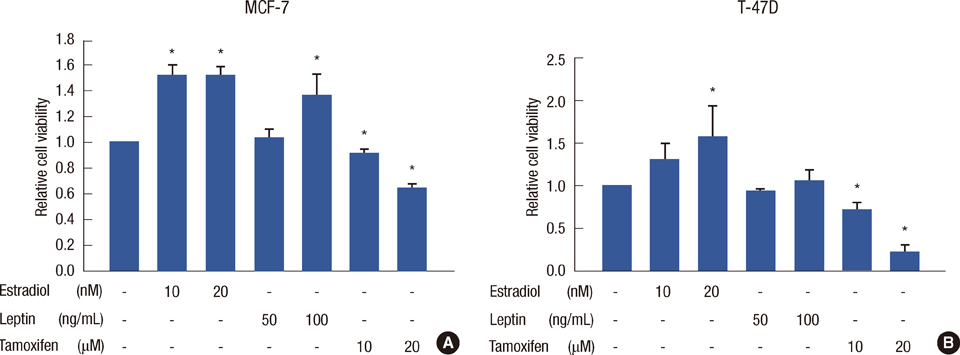
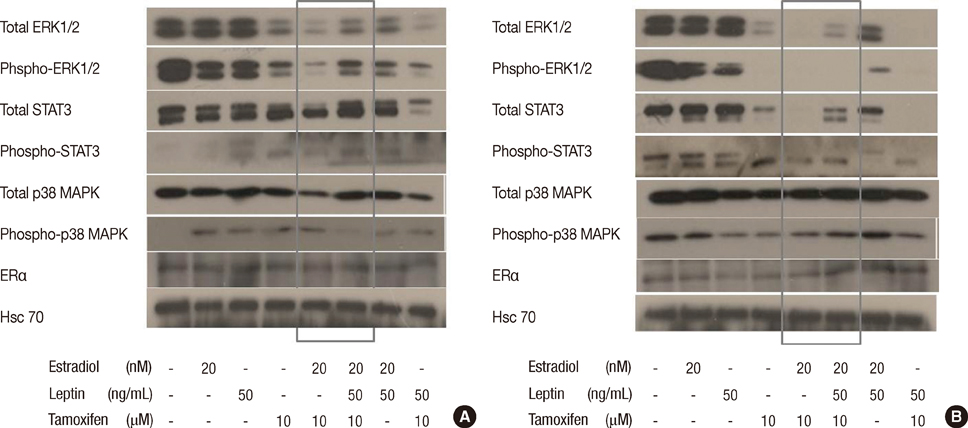
Leptin was expressed in MCF-7 and T-47D and had a proliferative effect on MCF-7 cells. Leptin significantly inhibited the antiestrogenic effect of tamoxifen in both cells only under beta-estradiol (E2) (20 nM) conditions. In MCF-7, the inhibitory effect against tamoxifen was a result from the activation of the ERK1/2 and STAT3 signal transduction pathway.
CONCLUSION
Leptin interferes with the effects of tamoxifen under E2 stimulated conditions in ER-positive breast cancer cells. These results imply that inhibition of leptin is expected to enhance the response to tamoxifen in ER-positive breast cancer cells, and, therefore, could be a promising way to overcome endocrine resistance.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Obesity, Diabetes, and Increased Cancer Progression
Dae-Seok Kim, Philipp E. Scherer
Diabetes Metab J. 2021;45(6):799-812. doi: 10.4093/dmj.2021.0077.
Reference
-
1. Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res Treat. 2007; 103:343–348.
Article2. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003; 95:1218–1226.
Article3. Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007; 14:189–206.
Article4. Pischon T, Nöthlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008; 67:128–145.
Article5. Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007; 67:2497–2507.
Article6. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995; 83:1263–1271.
Article7. Bjørbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997; 272:32686–32695.
Article8. Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun. 1998; 246:752–759.9. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006; 12:1447–1453.
Article10. Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review). Int J Oncol. 2002; 21:1285–1292.
Article11. Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003; 278:28668–28676.
Article12. Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Leptin directly stimulates aromatase activity in human luteinized granulosa cells. Mol Hum Reprod. 1999; 5:708–713.
Article13. Spicer LJ, Francisco CC. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 1997; 138:3374–3379.
Article14. Ghizzoni L, Barreca A, Mastorakos G, Furlini M, Vottero A, Ferrari B, et al. Leptin inhibits steroid biosynthesis by human granulosa-lutein cells. Horm Metab Res. 2001; 33:323–328.
Article15. Machinal-Quélin F, Dieudonné MN, Pecquery R, Leneveu MC, Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine. 2002; 18:179–184.
Article16. Bennett PA, Lindell K, Karlsson C, Robinson IC, Carlsson LM, Carlsson B. Differential expression and regulation of leptin receptor isoforms in the rat brain: effects of fasting and oestrogen. Neuroendocrinology. 1998; 67:29–36.
Article17. Lindell K, Bennett PA, Itoh Y, Robinson IC, Carlsson LM, Carlsson B. Leptin receptor 5'untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol Cell Endocrinol. 2001; 172:37–45.
Article18. Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin Cancer Res. 2004; 10:6466–6475.
Article19. Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004; 279:19908–19915.
Article20. Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin: a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002; 94:1704–1711.
Article21. Surmacz E. Obesity hormone leptin: a new target in breast cancer? Breast Cancer Res. 2007; 9:301.
Article22. Chen X, Zha X, Chen W, Zhu T, Qiu J, Røe OD, et al. Leptin attenuates the anti-estrogen effect of tamoxifen in breast cancer. Biomed Pharmacother. 2013; 67:22–30.
Article23. Binai NA, Damert A, Carra G, Steckelbroeck S, Löwer J, Löwer R, et al. Expression of estrogen receptor alpha increases leptin-induced STAT3 activity in breast cancer cells. Int J Cancer. 2010; 127:55–66.
Article24. Marsaud V, Gougelet A, Maillard S, Renoir JM. Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor alpha (ERalpha), differentially affect ERalpha extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol. 2003; 17:2013–2027.
Article25. Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003; 278:42660–42667.26. Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, et al. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem. 2004; 279:16495–16502.
Article27. Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998; 83:1059–1066.
Article28. Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000; 5:421–426.
Article29. Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006; 237:109–114.
Article30. Madaio RA, Spalletta G, Cravello L, Ceci M, Repetto L, Naso G. Overcoming endocrine resistance in breast cancer. Curr Cancer Drug Targets. 2010; 10:519–528.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leptin and Leptin Receptor Expression in Breast Cancer
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- The Role of Long Noncoding RNAs in Antiestrogen Resistance in Breast Cancer: An Overview and Update
- Expression of Leptin, Leptin Receptor, Adiponectin, and Adiponectin Receptor in Ductal Carcinoma In Situ and Invasive Breast Cancer
- Effects of the Expression of Leptin and Leptin Receptor (OBR) on the Prognosis of Early-stage Breast Cancers