J Breast Cancer.
2011 Jun;14(2):96-103. 10.4048/jbc.2011.14.2.96.
Expression of Leptin, Leptin Receptor, Adiponectin, and Adiponectin Receptor in Ductal Carcinoma In Situ and Invasive Breast Cancer
- Affiliations
-
- 1Department of Surgery, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 2Department of Molecular Biology, Kyung Hee University College of Pharmacy, Seoul, Korea.
- 3Department of Pathology, Catholic University of Daegu School of Medicine, Daegu, Korea. ap510@cu.ac.kr
- KMID: 2286507
- DOI: http://doi.org/10.4048/jbc.2011.14.2.96
Abstract
- PURPOSE
Adipocytokines, such as leptin, resistin, and adiponectin, are associated with obesity and breast cancer. Several studies have indicated that adipocytokines may influence tumor growth or differentiation. The aims of this study were to determine the expression of leptin, leptin receptor (ObR), adiponectin and adiponectin receptor (AdipoR) in human breast cancer, to evaluate their prognostic significance in the breast cancer.
METHODS
Specimens from 198 patients with primary breast cancer were enrolled, and representative paraffin tumor blocks were selected for constructing tissue microarrarys (TMA). Immunohistochemical staining for leptin, ObR, adiponectin, and AdipoR was performed using TMA, and the clinicopathologic characteristics were evaluated from the patient's medical records.
RESULTS
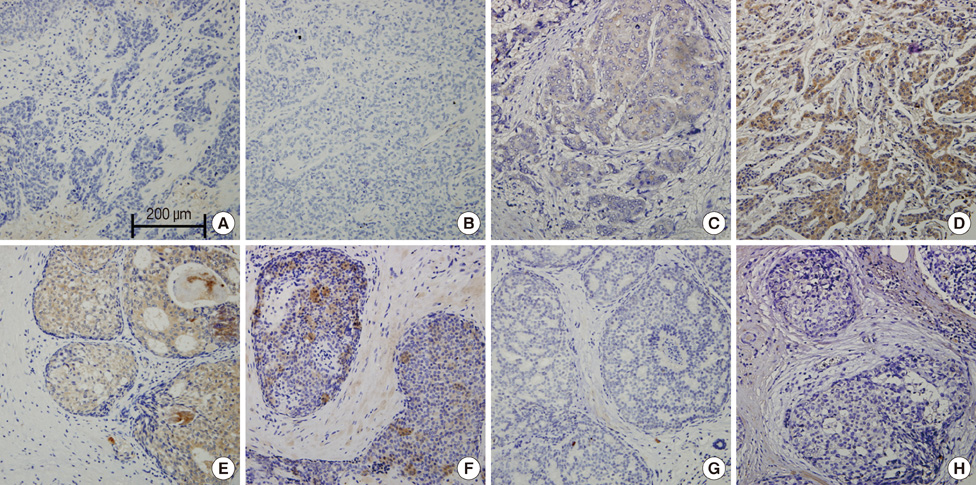
Stage 0 breast cancer accounted for 41 cases, and 157 cases were invasive cancer. Positive rates of leptin and ObR expression in the ductal carcinoma in situ (DCIS) group were significantly higher than those of the invasive cancer group (97.4% vs. 34.0%, p<0.001; 74.4% vs. 29.8%, p<0.001). However, positive rates of adiponectin and AdipoR expression in the invasive cancer group were significantly higher than those in the DCIS group (53.7% vs. 33.3%, p=0.024; 59.9% vs. 26.3%, p<0.001). High leptin expression was significantly associated with high Ki-67 expression (p=0.016). High adiponectin expression was significantly correlated with smaller tumor size (p=0.001).
CONCLUSION
We suggest that losses of leptin and ObR expression could be associated with invasive cancer, whereas high adiponectin and AdipoR expression may be associated with breast cancer invasiveness.
Keyword
MeSH Terms
Figure
Reference
-
1. Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res Treat. 2007. 103:343–348.
Article2. Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003. 45:1–16.
Article3. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003. 95:1218–1226.
Article4. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004. 363:1346–1353.
Article5. Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009. 20:193–201.
Article6. Housa D, Housová J, Vernerová Z, Haluzík M. Adipocytokines and cancer. Physiol Res. 2006. 55:233–244.
Article7. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006. 207:12–22.
Article8. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.
Article9. Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002. 94:1704–1711.
Article10. Jardé T, Caldefie-Chézet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008. 53:484–487.
Article11. Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004. 10:4325–4331.
Article12. Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000. 5:421–426.
Article13. Huang L, Li C. Leptin: a multifunctional hormone. Cell Res. 2000. 10:81–92.
Article14. Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008. 68:9712–9722.
Article15. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006. 12:1447–1453.
Article16. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995. 83:1263–1271.
Article17. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.
Article18. Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003. 9:5699–5704.19. Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004. 89:1102–1107.
Article20. Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007. 13:332–339.
Article21. Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009. 28:2621–2633.
Article22. McMurtry V, Simeone AM, Nieves-Alicea R, Tari AM. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin Exp Metastasis. 2009. 26:197–204.
Article23. Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007. 14:189–206.
Article24. Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem. 2006. 281:26320–26328.
Article25. Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006. 98:121–132.
Article26. Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008. 20:971–977.
Article27. Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res. 2007. 39:9–13.
Article28. Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol. 2007. 24:361–366.
Article29. Pfeiler G, Treeck O, Wenzel G, Goerse R, Hartmann A, Schmitz G, et al. Influence of insulin resistance on adiponectin receptor expression in breast cancer. Maturitas. 2009. 63:253–256.
Article30. Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010. 119:367–377.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer
- Correlations of Leptin, Adiponectin and Leptin/Adiponectin Ratio with Metabolic Disorders in the Childhood Obesity
- Leptin and Leptin Receptor Expression in Breast Cancer
- Puberty and Gender Differences of Plasma Leptin, Adiponectin Levels, and Leptin/Adiponectin Ratio
- The relationship between leptin adiponectin ratio and insulin resistance in healthy children