Obstet Gynecol Sci.
2016 Jul;59(4):279-285. 10.5468/ogs.2016.59.4.279.
Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University School of Medicine, Seoul, Korea. onco@ewha.ac.kr
- KMID: 2329048
- DOI: http://doi.org/10.5468/ogs.2016.59.4.279
Abstract
OBJECTIVE
Decreased adiponectin and increased leptin plasma concentrations are believed to be associated with the occurrence and progression of cancers such as endometrial cancer and breast cancer. The aim of this study was to explore the association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer.
METHODS
For patients with ovarian cancer and the control group, adiponectin and leptin levels were measured; anthropometric data were obtained during a chart review. Statistical comparisons between groups were analyzed using the Student's t-test; correlations were confirmed using the Pearson correlation.
RESULTS
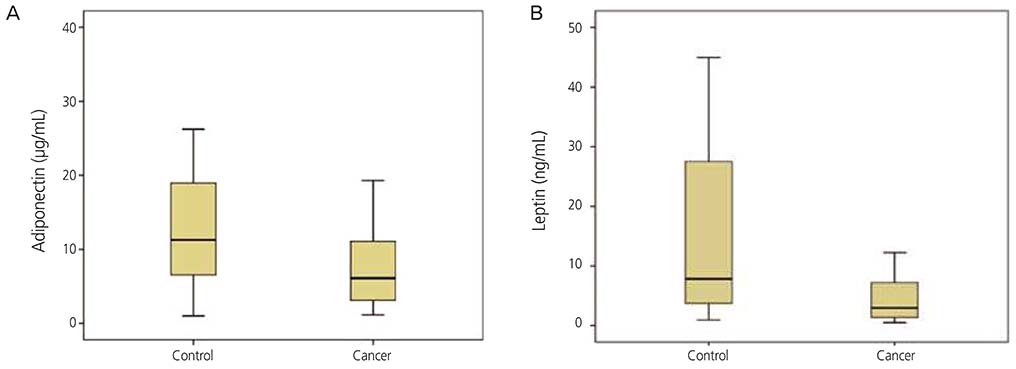
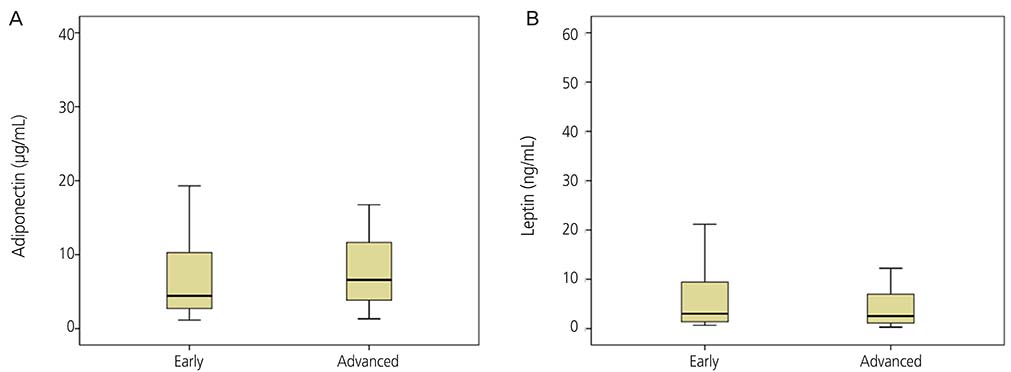
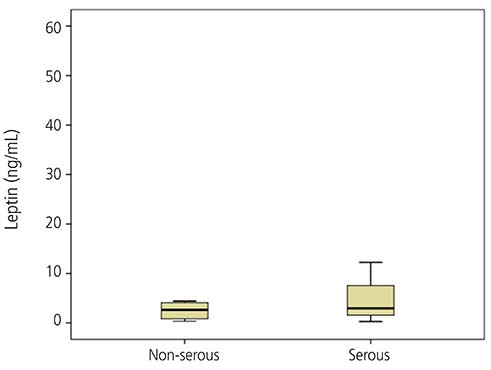
The mean adiponectin and leptin concentrations in patients with ovarian cancer were lower than those of the control group (8.25 vs. 11.44 µg/mL, respectively; P=0.026) (7.09 vs. 15.4 ng/mL, respectively; P=0.001). However, there was no significant difference in adiponectin and leptin levels between early-stage (I/II) and advanced-stage (III/IV) disease (P=0.078).
CONCLUSION
Compared with other gynecological cancers, the level of adiponectin and leptin were decreased in ovarian cancer that may have some diagnostic value; additional study to elucidate the function of these two hormones in the development of ovarian carcinogenesis is necessitated.
Keyword
MeSH Terms
Figure
Reference
-
1. Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, et al. A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013; 119:3454–3461.2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.3. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.4. Gnacińska M, Malgorzewicz S, Stojek M, Lysiak-Szydlowska W, Sworczak K. Role of adipokines in complications related to obesity: a review. Adv Med Sci. 2009; 54:150–157.5. Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, et al. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. J Clin Endocrinol Metab. 2007; 92:2665–2671.6. Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95 2. Endocr Relat Cancer. 2007; 14:713–720.7. Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009; 15:3265–3276.8. Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007; 9:282–289.9. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007; 380:24–30.10. Owecki M, Nikisch E, Miczke A, Pupek-Musialik D, Sowinski J. Leptin, soluble leptin receptors, free leptin index, and their relationship with insulin resistance and BMI: high normal BMI is the threshold for serum leptin increase in humans. Horm Metab Res. 2010; 42:585–589.11. Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996; 81:4406–4413.12. Kimura E, Matsumoto K, Samori T, Kato S, Kawahara T. One-step enzyme-linked immunosorbent assay (ELISA) for measurement of serum free leptin. Clin Chim Acta. 2000; 296:45–57.13. Ma Y, Liu Z, Zhang Y, Lu B. Serum leptin, adiponectin and endometrial cancer risk in Chinese women. J Gynecol Oncol. 2013; 24:336–341.14. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000; 70:209–262.15. Minatoya M, Kutomi G, Shima H, Asakura S, Otokozawa S, Ohnishi H, et al. Relation of serum adiponectin levels and obesity with breast cancer: a Japanese case-control study. Asian Pac J Cancer Prev. 2014; 15:8325–8330.16. Reinehr T, Woelfle J, Wiegand S, Karges B, Meissner T, Nagl K, et al. Leptin but not adiponectin is related to type 2 diabetes mellitus in obese adolescents. Pediatr Diabetes. 2015; 04. 16. DOI: 10.1111/pedi.12276.17. Noda T, Kikugawa T, Tanji N, Miura N, Asai S, Higashiyama S, et al. Long term exposure to leptin enhances the growth of prostate cancer cells. Int J Oncol. 2015; 46:1535–1542.18. Duan X, Tang P, Zhang H, Yu Z. Expression of leptin and adiponectin in esophageal squamous cell carcinoma and their clinical significance. Zhonghua Zhong Liu Za Zhi. 2014; 36:839–843.19. Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013; 43:394–403.20. Tanaka M, Suganami T, Kim-Saijo M, Toda C, Tsuiji M, Ochi K, et al. Role of central leptin signaling in the starvation-induced alteration of B-cell development. J Neurosci. 2011; 31:8373–8380.21. Luhn P, Dallal CM, Weiss JM, Black A, Huang WY, Lacey JV Jr, et al. Circulating adipokine levels and endometrial cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2013; 22:1304–1312.22. Grabowski JP, Markowska A, Markowska J. Evaluation of leptin serum concentrations during surgery and first-line chemotherapy in primary epithelial ovarian cancer patients. Contemp Oncol (Pozn). 2014; 18:318–322.23. Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005; 102:7677–7682.24. Macciò A, Madeddu C, Massa D, Mudu MC, Lusso MR, Gramignano G, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancerrelated anemia. Blood. 2005; 106:362–367.25. Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008; 14:1065–1072.26. Nick AM, Sood AK. The ROC 'n' role of the multiplex assay for early detection of ovarian cancer. Nat Clin Pract Oncol. 2008; 5:568–569.27. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996; 382:250–252.28. Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP Diet and Health Study. Int J Cancer. 2012; 131:938–948.29. Hoskins WJ. Prospective on ovarian cancer: why prevent? J Cell Biochem Suppl. 1995; 23:189–199.30. Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002; 147:173–180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Puberty and Gender Differences of Plasma Leptin, Adiponectin Levels, and Leptin/Adiponectin Ratio
- The relationship between leptin adiponectin ratio and insulin resistance in healthy children
- Correlations of Leptin, Adiponectin and Leptin/Adiponectin Ratio with Metabolic Disorders in the Childhood Obesity
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus
- Association of Adipokines with Development and Progression of Nonalcoholic Fatty Liver Disease