Immune Netw.
2017 Apr;17(2):110-115. 10.4110/in.2017.17.2.110.
Characterization of the Indirubin Derivative LDD970 as a Small Molecule Aurora Kinase A Inhibitor in Human Colorectal Cancer Cells
- Affiliations
-
- 1College of Pharmacy and Research Institute of Pharmaceutical Sciences, Gyeongsang National University, Jinju 52828, Korea. syhan@gnu.ac.kr
- 2School of life Sciences, Gwangju Institute of Science & Technology, Gwangju 61186, Korea.
- KMID: 2376868
- DOI: http://doi.org/10.4110/in.2017.17.2.110
Abstract
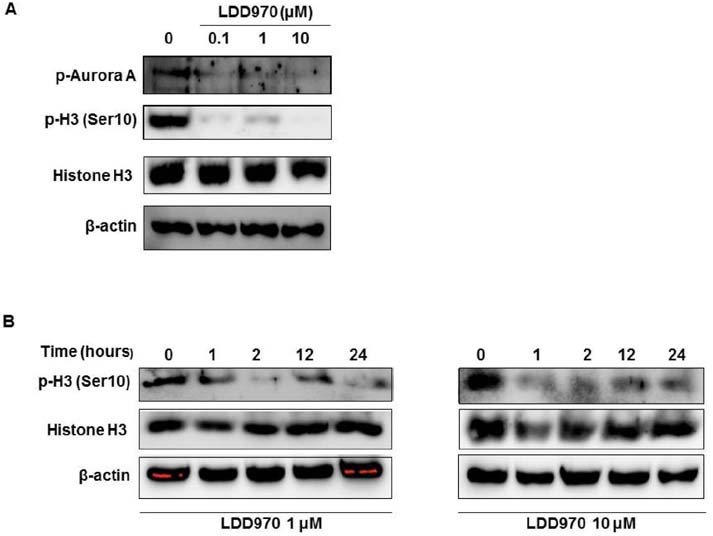
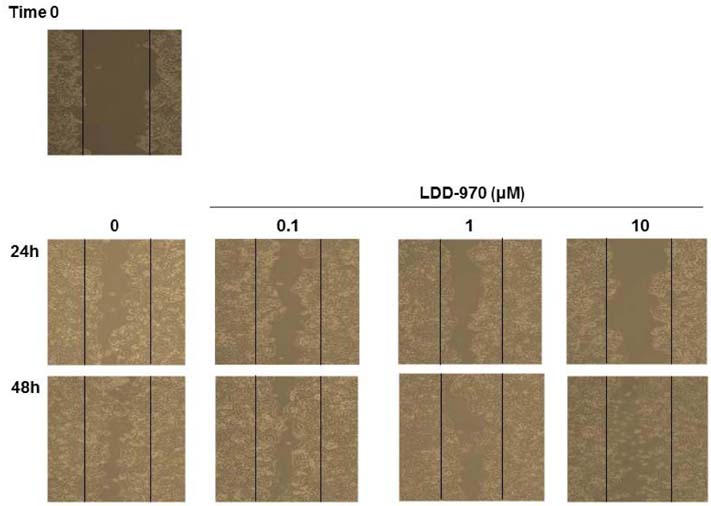
- Aurora kinase A plays an essential role in mitosis including chromosome separation and cytokinesis. Aberrant expression and activity of Aurora kinase A is associated with numerous malignancies including colorectal cancer followed by poor prognosis. The aim of this study is to determine the inhibitory effects of LDD970, an indirubin derivative, on Aurora kinase A in HT29 colorectal cancer cells. In vitro kinase assay revealed that, LDD970 inhibited levels of activated Aurora kinase A (ICâ‚…â‚€=0.37 mM). The inhibitory effects of LDD970 on Aurora kinase A, autophosphorylation and phosphorylation of histone H3 (Ser10), were confirmed by immunoblot analysis. Moreover, LDD970 inhibited migration of HT29 cells and upregulated apoptosis-related protein cleaved PARP. In cell viability assay, LDD970 was observed to suppress HT29 cell growth (GIâ‚…â‚€=4.22 µM). Although further studies are required, results of the present study suggest that LDD970 provide a valuable insight into small molecule indirubin derivative for therapeutic potential in human colorectal cancer.
MeSH Terms
Figure
Reference
-
1. Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999; 112:3591–3601.
Article2. Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Aurora kinases in head and neck cancer. Lancet Oncol. 2013; 14:e425–e435.
Article3. Dodson CA, Bayliss R. Activation of Aurora-A kinase by protein partner binding and phosphorylation are independent and synergistic. J Biol Chem. 2012; 287:1150–1157.
Article4. Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006; 16:356–366.
Article5. Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004; 10:2065–2071.
Article6. Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998; 17:3052–3065.
Article7. Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003; 116:3677–3685.
Article8. Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001; 20:3021–3027.
Article9. Van HA, Goodrich DW, Allis CD, Brinkley BR, Mancini MA. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J Cell Sci. 1998; 111:3497–3506.
Article10. Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002; 22:874–885.
Article11. Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999; 1:60–67.
Article12. Moon MJ, Lee SK, Lee JW, Song WK, Kim SW, Kim JI, Cho C, Choi SJ, Kim YC. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg Med Chem. 2006; 14:237–246.
Article13. Gaboriaud-Kolar N, Vougogiannopoulou K, Skaltsounis AL. Indirubin derivatives: a patent review (2010-present). Expert Opin Ther Pat. 2015; 25:583–593.14. Chung HJ, Kamli MR, Lee HJ, Ha JD, Cho SY, Lee J, Kong JY, Han SY. Discovery of quinolinone derivatives as potent FLT3 inhibitors. Biochem Biophys Res Commun. 2014; 445:561–565.
Article15. Goshima G, Vale RD. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol. 2003; 162:1003–1016.16. van Vuuren RJ, Visagie MH, Theron AE, Joubert AM. Antimitotic drugs in the treatment of cancer. Cancer Chemother Pharmacol. 2015; 76:1101–1112.
Article17. Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ, Ray ET, Sells TB, Stringer B, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Bolen JB, Claiborne CF. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007; 104:4106–4111.
Article18. D'Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci. 2001; 114:3771–3778.19. Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, Nahar S, Zheng M, Bandi M, Carrasco RD, Raje N, Munshi N, Richardson P, Anderson KC. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010; 115:5202–5213.
Article20. Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M. Aurora kinase inhibitors: progress towards the clinic. Invest New Drugs. 2012; 30:2411–2432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Discovery of an Indirubin Derivative as a Novel c-Met Kinase Inhibitor with In Vitro Anti-Tumor Effects
- IMMUNOHISTOCHEMICAL STUDY OF AURORA-2 KINASE IN THE ORAL SQUAMOUS CELL CARCINOMA
- Elevated Aurora Kinase A Protein Expression in Diabetic Skin Tissue
- Regulation of Phosphorylation of Glycogen Synthase Kinase 3α and the Correlation with Sperm Motility in Human
- Novel SIRT Inhibitor, MHY2256, Induces Cell Cycle Arrest, Apoptosis, and Autophagic Cell Death in HCT116 Human Colorectal Cancer Cells