Nat Prod Sci.
2017 Mar;23(1):1-4. 10.20307/nps.2017.23.1.1.
Sinensetin Inhibits Interleukin-6 in Human Mast Cell - 1 Via Signal Transducers and Activators of the Transcription 3 (STAT3) and Nuclear Factor Kappa B (NF-κB) Pathways
- Affiliations
-
- 1College of Pharmacy, Dongguk University-Seoul, 32, Dongguk-lo, Ilsandong-gu, Goyang Gyeonggi-do 410-820, Republic of Korea. f2744@dongguk.edu
- KMID: 2376491
- DOI: http://doi.org/10.20307/nps.2017.23.1.1
Abstract
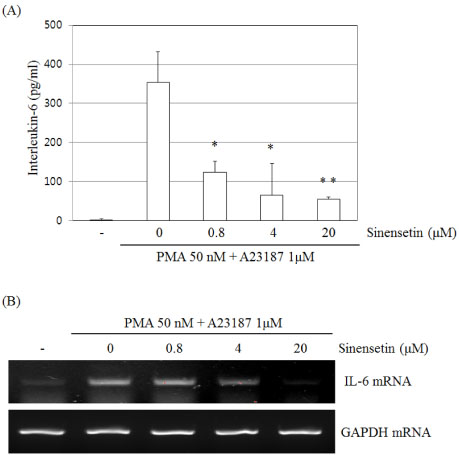
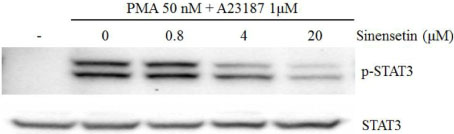
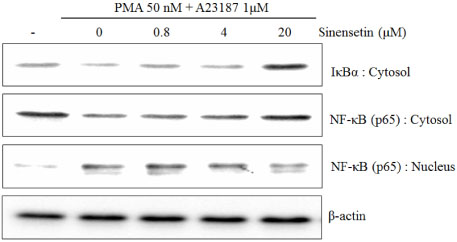
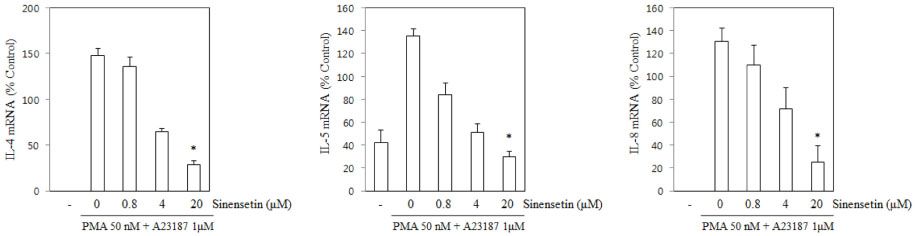
- Sinensetin, a pentamethoxyflavone, is known to exert various pharmacological activities including anti-angiogenesis, anti-diabetic and anti-inflammatory activities. However, its effects on the human mast cell - 1 (HMC-1) mediated inflammatory mechanism remain unknown. To explore the mediator and cellular inflammatory response of sinensetin, we examined its influence on phorbol 12-myristate 13-acetate (PMA) plus A23187 induced inflammatory mediator production in a human mast cell line. In this study, interleukin (IL)-6 production was measured using the enzyme-linked immunosorbent assay and reverse transcription polymerase chain reaction. Sinensetin inhibited PMA plus A23187 induced IL-6 production in a dose-dependent manner as well as IL-4, IL-5 and IL-8 mRNA expression. Furthermore, sinensetin inhibited signal transducer and activator of transcription 3 (STAT3) phosphorylation, suggesting that sinensetin inhibits the production of inflammatory mediators by blocking STAT3 phosphorylation. Moreover, sinensetin was found to inhibit nuclear factor kappa B activation. These findings suggest that sinensetin may be involved in the regulation of mast cell-mediated inflammatory responses.
Keyword
MeSH Terms
-
Calcimycin
Enzyme-Linked Immunosorbent Assay
Humans*
Interleukin-4
Interleukin-5
Interleukin-6*
Interleukin-8
Interleukins
Mast Cells*
NF-kappa B*
Phosphorylation
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
STAT3 Transcription Factor
Transducers*
Calcimycin
Interleukin-4
Interleukin-5
Interleukin-6
Interleukin-8
Interleukins
NF-kappa B
RNA, Messenger
STAT3 Transcription Factor
Figure
Reference
-
1. Wolff B, Burns AR, Middleton J, Rot A. J Exp Med. 1998; 188:1757–1762.2. Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. J Exp Med. 1998; 188:1751–1756.3. Baggiolini M, Loetscher P, Moser B. Int J Immunopharmacol. 1995; 17:103–108.4. Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J, Albano E, Ingelman-Sundberg M, Badger TM. Exp Biol Med (Maywood). 2008; 233:344–355.5. Conti P, Kempuraj D, Di Gioacchino M, Boucher W, Letourneau R, Kandere K, Barbacane RC, Reale M, Felaco M, Frydas S, Theoharides TC. Allergy Asthma Proc. 2002; 23:331–335.
Article6. Kikuchi T, Ishida S, Kinoshita T, Sakuma S, Sugawara N, Yamashita T, Koike K. Cytokine. 2002; 20:200–209.7. Castellani ML, Ciampoli C, Felaco M, Tetè S, Conti CM, Salini V, De Amicis D, Orso C, Antinolfi PL, Caraffa A, Cerulli G, Boscolo P, Theoharides TC, Conti P, Kepuraj D. Clin Invest Med. 2008; 31:E362–E372.8. Nakahata T, Toru H. Int J Hematol. 2002; 75:350–356.9. Kim SH, Kim SH, Kim SH, Moon JY, Park WH, Kim CH, Shin TY. Biol Pharm Bull. 2006; 29:494–498.10. Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Immunol Rev. 2007; 217:65–78.11. Ihle JN. Cell. 1996; 84:331–334.12. Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Proc Natl Acad Sci U S A. 1997; 94:3801–3804.13. Zhong Z, Wen Z, Darnell JE Jr. Science. 1994; 264:95–98.14. Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. J Biol Chem. 2007; 282:9358–9363.15. Laavola M, Nieminen R, Yam MF, Sadikun A, Asmawi MZ, Basir R, Welling J, Vapaatalo H, Korhonen R, Moilanen E. Planta Med. 2012; 78:779–786.16. Shin HS, Kang SI, Yoon SA, Ko HC, Kim SJ. Biosci Biotechnol Biochem. 2012; 76:847–849.17. Kishimoto T. Annu Rev Immunol. 2005; 23:1–21.18. Quintanilla-Martinez L, Kremer M, Specht K, Calzada-Wack J, Nathrath M, Schaich R, Höfler H, Fend F. Am J Pathol. 2003; 162:1449–1461.19. Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Cell. 1994; 77:63–71.20. Darnell JE Jr, Kerr IM, Stark GR. Science. 1994; 264:1415–1421.21. Murray PJ. J Immunol. 2007; 178:2623–2629.22. Hodge DR, Hurt EM, Farrar WL. Eur J Cancer. 2005; 41:2502–2512.23. Lee MM, Chui RK, Tam IY, Lau AH, Wong YH. J Immunol. 2012; 189:5266–5276.24. Fleischer A, Ghadiri A, Dessauge F, Duhamel M, Rebollo MP, Alvarez-Franco F, Rebollo A. Mol Immunol. 2006; 43:1065–1079.25. Lappas M, Permezel M, Georgiou HM, Rice GE. Biol Reprod. 2002; 67:668–673.26. Ivanenkov YA, Balakin KV, Tkachenko SE. Drugs R D. 2008; 9:397–434.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Enhanced Protein Expression of Signal Transducer and Activator of Transcription 3 and Protein Kinase Substrate p36 in Hepatocellular Carcinoma
- Controls of Nuclear Factor-Kappa B Signaling Activity by 5’-AMP-Activated Protein Kinase Activation With Examples in Human Bladder Cancer Cells
- The Clinical Significance of STAT3 and STAT5 Activation in Renal Cell Carcinoma
- alpha-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-kappaB in H. pylori-Infected Gastric Epithelial AGS Cells