Yonsei Med J.
2016 Jan;57(1):260-264. 10.3349/ymj.2016.57.1.260.
alpha-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-kappaB in H. pylori-Infected Gastric Epithelial AGS Cells
- Affiliations
-
- 1Department of Food and Nutrition, Brain Korea 21 PLUS Project, College of Human Ecology, Yonsei University, Seoul, Korea. kim626@yonsei.ac.kr
- 2Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2466379
- DOI: http://doi.org/10.3349/ymj.2016.57.1.260
Abstract
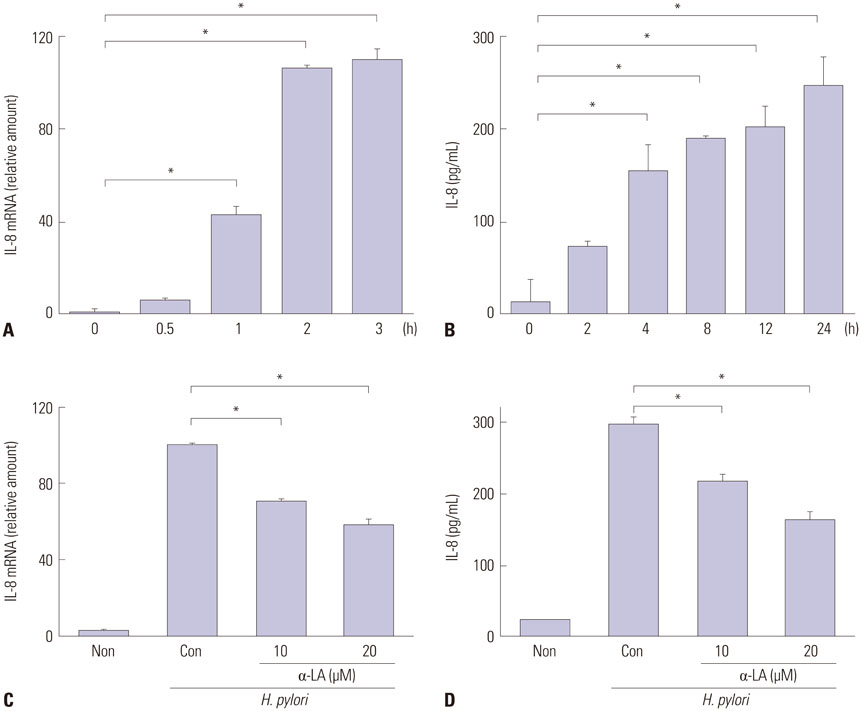
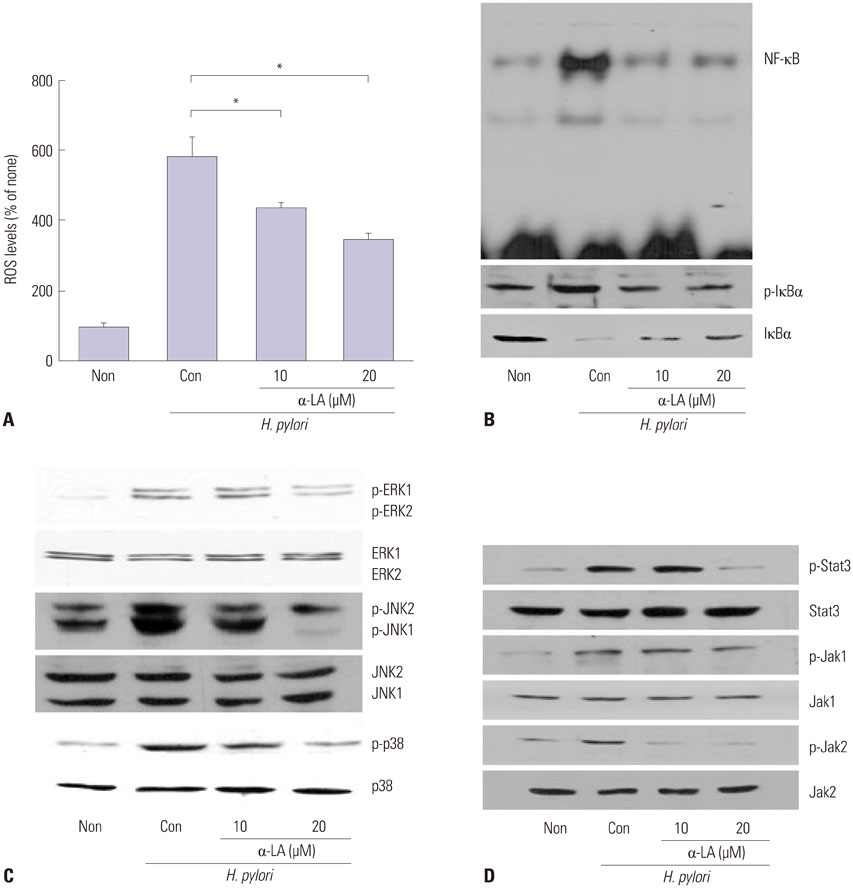
- The epithelial cytokine response, associated with reactive oxygen species (ROS), is important in Helicobacter pylori (H. pylori)-induced inflammation. H. pylori induces the production of ROS, which may be involved in the activation of mitogen-activated protein kinases (MAPK), janus kinase/signal transducers and activators of transcription (Jak/Stat), and oxidant-sensitive transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB), and thus, expression of interleukin-8 (IL-8) in gastric epithelial cells. alpha-lipoic acid, a naturally occurring thiol compound, is a potential antioxidant. It shows beneficial effects in treatment of oxidant-associated diseases including diabetes. The present study is purposed to investigate whether alpha-lipoic acid inhibits expression of inflammatory cytokine IL-8 by suppressing activation of MAPK, Jak/Stat, and NF-kappaB in H. pylori-infected gastric epithelial cells. Gastric epithelial AGS cells were pretreated with or without alpha-lipoic acid for 2 h and infected with H. pylori in a Korean isolate (HP99) at a ratio of 300:1. IL-8 mRNA expression was analyzed by RT-PCR analysis. IL-8 levels in the medium were determined by enzyme-linked immunosorbent assay. NF-kappaB-DNA binding activity was determined by electrophoretic mobility shift assay. Phospho-specific and total forms of MAPK and Jak/Stat were assessed by Western blot analysis. ROS levels were determined using dichlorofluorescein fluorescence. As a result, H. pylori induced increases in ROS levels, mRNA, and protein levels of IL-8, as well as the activation of MAPK [extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase 1/2 (JNK1/2), p38], Jak/Stat (Jak1/2, Stat3), and NF-kappaB in AGS cells, which was inhibited by alpha-lipoic acid. In conclusion, alpha-lipoic acid may be beneficial for prevention and/or treatment of H. pylori infection-associated gastric inflammation.
Keyword
MeSH Terms
-
Enzyme-Linked Immunosorbent Assay
Epithelial Cells/metabolism
Gastric Mucosa/*drug effects/metabolism/microbiology
Gene Expression Regulation, Bacterial
Helicobacter Infections/immunology/*metabolism
Helicobacter pylori/drug effects/*pathogenicity
Humans
Interleukin-8/genetics/*metabolism
JNK Mitogen-Activated Protein Kinases
Janus Kinase 1
Mitogen-Activated Protein Kinases/*biosynthesis
NF-kappa B/*metabolism
RNA, Messenger/isolation & purification/metabolism
Reactive Oxygen Species/metabolism
STAT3 Transcription Factor
Stomach/metabolism/*microbiology
Thioctic Acid/*pharmacology
JNK Mitogen-Activated Protein Kinases
Janus Kinase 1
Interleukin-8
Mitogen-Activated Protein Kinases
NF-kappa B
RNA, Messenger
Reactive Oxygen Species
STAT3 Transcription Factor
Thioctic Acid
Figure
Reference
-
1. Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002; 2:28–37.
Article2. Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995; 48:133–136.
Article3. Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004; 84:49–62.
Article4. Kim H. Oxidative stress in Helicobacter pylori-induced gastric cell injury. Inflammopharmacology. 2005; 13:63–74.
Article5. O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002; 109:S121–S131.6. Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001; 36:706–716.
Article7. Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003; 48:257–265.8. Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007; 282:31460–31468.
Article9. Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, et al. Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006; 9:101–110.
Article10. Fujita H, Shiosaka M, Ogino T, Okimura Y, Utsumi T, Sato EF, et al. Alpha-lipoic acid suppresses 6-hydroxydopamine-induced ROS generation and apoptosis through the stimulation of glutathione synthesis but not by the expression of heme oxygenase-1. Brain Res. 2008; 1206:1–12.
Article11. Kishi Y, Schmelzer JD, Yao JK, Zollman PJ, Nickander KK, Tritschler HJ, et al. Alpha-lipoic acid: effect on glucose uptake, sorbitol pathway, and energy metabolism in experimental diabetic neuropathy. Diabetes. 1999; 48:2045–2051.
Article12. Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004; 11:1135–1146.
Article13. Aly HA, Lightfoot DA, El-Shemy HA. Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem Biol Interact. 2009; 182:112–118.
Article14. Zhang H, Jia H, Liu J, Ao N, Yan B, Shen W, et al. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson's disease. J Cell Mol Med. 2010; 14:215–225.
Article15. Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002; 33:83–93.
Article16. Byun E, Lim JW, Kim JM, Kim H. α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells. Mediators Inflamm. 2014; 2014:380830.17. Cho SO, Lim JW, Kim KH, Kim H. Diphenyleneiodonium inhibits the activation of mitogen-activated protein kinases and the expression of monocyte chemoattractant protein-1 in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm Res. 2011; 60:501–507.
Article18. Cha B, Lim JW, Kim KH, Kim H. 15-deoxy-D12,14-prostaglandin J2 suppresses RANTES expression by inhibiting NADPH oxidase activation in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol. 2011; 62:167–174.19. Lee SE, Lim JW, Kim JM, Kim H. Anti-inflammatory mechanism of polyunsaturated fatty acids in Helicobacter pylori-infected gastric epithelial cells. Mediators Inflamm. 2014; 2014:128919.20. Ito A, Uehara T, Tokumitsu A, Okuma Y, Nomura Y. Possible involvement of cytochrome c release and sequential activation of caspases in ceramide-induced apoptosis in SK-N-MC cells. Biochim Biophys Acta. 1999; 1452:263–274.
Article21. Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002; 285:1–24.
Article22. Bronte-Tinkew DM, Terebiznik M, Franco A, Ang M, Ahn D, Mimuro H, et al. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009; 69:632–639.
Article23. Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007; 67:2497–2507.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- Nucleotide Binding Oligomerization Domain 1 Is an Essential Signal Transducer in Human Epithelial Cells Infected with Helicobacter pylori That Induces the Transepithelial Migration of Neutrophils
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- Taurine-conjugated Bile Acids Induce Nuclear Factor-Kappa B Mediated Interleukin-8 Activation in Gastric Epithelial Cell Lines