J Breast Cancer.
2015 Jun;18(2):112-118. 10.4048/jbc.2015.18.2.112.
Isocryptotanshinone Induced Apoptosis and Activated MAPK Signaling in Human Breast Cancer MCF-7 Cells
- Affiliations
-
- 1State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China. chenxiu0725@yeah.net
- KMID: 2374525
- DOI: http://doi.org/10.4048/jbc.2015.18.2.112
Abstract
- PURPOSE
Isocryptotanshinone (ICTS) is a natural bioactive product that is isolated from the roots of the widely used medical herb Salvia miltiorrhiza. However, few reports exist on the mechanisms underlying the therapeutic effects of ICTS. Here, we report that ICTS has anticancer activity and describe the mechanism underlying this effect.
METHODS
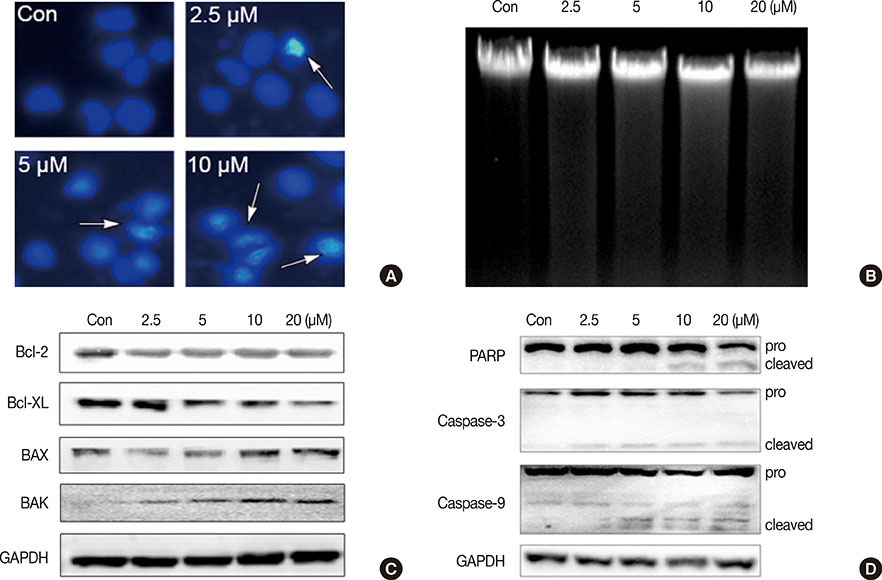
The antiproliferative effect of ICTS was determined using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) and clonogenic assays. The effect of ICTS on the cell cycle was measured using flow cytometry. Apoptosis was determined by Hoechst 33342 staining, DNA fragmentation assays, and Western blotting for apoptotic proteins. Finally, the effect of ICTS on mitogen-activated protein kinases (MAPKs) was determined by Western blotting.
RESULTS
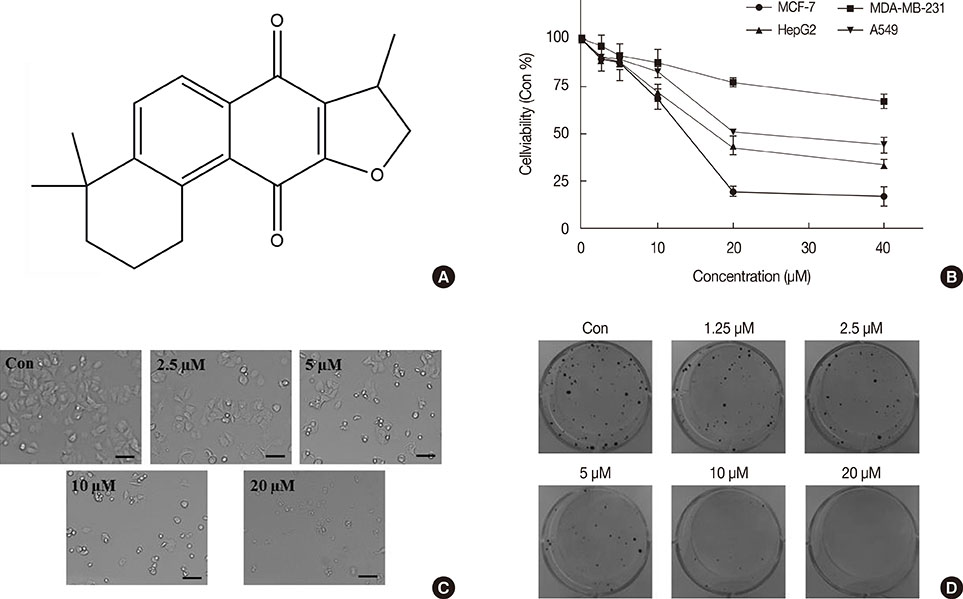
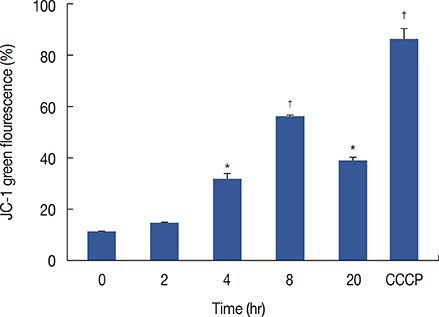
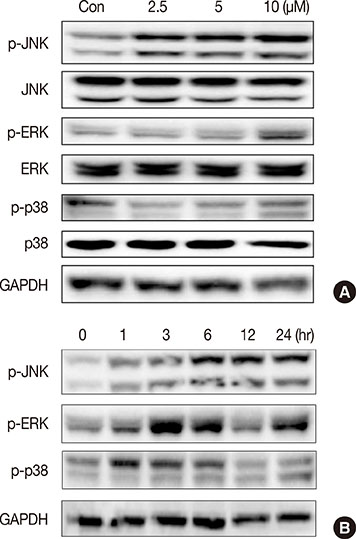
ICTS significantly inhibited proliferation of MCF-7 and MDA-MB-231 human breast cancer cells, HepG2 human liver cancer cells, and A549 human lung cancer cells in vitro. Among the tested cell lines, MCF-7 cells showed the highest sensitivity to ICTS. ICTS significantly inhibited colony formation by MCF-7 cells. Furthermore, exposure of MCF-7 cells to ICTS induced cell cycle arrest at the G1 phase and decreased mitochondrial membrane potential. Hoechst 33342 staining and Western blot analysis for apoptotic proteins suggested that ICTS induced apoptosis in MCF-7 cells. In addition, ICTS activated MAPK signaling in MCF-7 cells by inducing time- and concentration-dependent phosphorylation of JNK, ERK, and p38 MAPK.
CONCLUSION
Our results suggest that ICTS inhibited MCF-7 cell proliferation by inducing apoptosis and activating MAPK signaling pathways.
MeSH Terms
-
Apoptosis*
Blotting, Western
Breast Neoplasms*
Cell Cycle
Cell Cycle Checkpoints
Cell Line
DNA Fragmentation
Flow Cytometry
G1 Phase
Hep G2 Cells
Humans
Liver Neoplasms
Lung Neoplasms
MCF-7 Cells*
Membrane Potential, Mitochondrial
Mitogen-Activated Protein Kinases
p38 Mitogen-Activated Protein Kinases
Phosphorylation
Salvia miltiorrhiza
Mitogen-Activated Protein Kinases
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Chaveli-López B. Oral toxicity produced by chemotherapy: a systematic review. J Clin Exp Dent. 2014; 6:e81–e90.
Article2. Saha SK, Khuda-Bukhsh AR. Molecular approaches towards development of purified natural products and their structurally known derivatives as efficient anti-cancer drugs: current trends. Eur J Pharmacol. 2013; 714:239–248.
Article3. Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011; 6:27.
Article4. Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, et al. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007; 245:269–274.
Article5. Griffin C, Karnik A, McNulty J, Pandey S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol Cancer Ther. 2011; 10:57–68.
Article6. Badr CE, Van Hoppe S, Dumbuya H, Tjon-Kon-Fat LA, Tannous BA. Targeting cancer cells with the natural compound obtusaquinone. J Natl Cancer Inst. 2013; 105:643–653.
Article7. Zhang Y, Jiang P, Ye M, Kim SH, Jiang C, Lü J. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int J Mol Sci. 2012; 13:13621–13666.
Article8. Kakisawa H, Hayashi T, Yamazaki T. Structures of isotanshinones. Tetrahedron Lett. 1969; 10:301–304.
Article9. Han YM, Oh H, Na M, Kim BS, Oh WK, Kim BY, et al. PTP1B inhibitory effect of abietane diterpenes isolated from Salvia miltiorrhiza. Biol Pharm Bull. 2005; 28:1795–1797.
Article10. Chen X, Guo J, Bao J, Lu J, Wang Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev. 2014; 34:768–794.
Article11. Chen W, Liu L, Luo Y, Odaka Y, Awate S, Zhou H, et al. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev Res (Phila). 2012; 5:778–787.
Article12. Park IJ, Kim MJ, Park OJ, Choe W, Kang I, Kim SS, et al. Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells. Apoptosis. 2012; 17:248–257.
Article13. Park IJ, Yang WK, Nam SH, Hong J, Yang KR, Kim J, et al. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis. 2014; 19:615–628.
Article14. Wei H, Zhang X, Wu G, Yang X, Pan S, Wang Y, et al. Chalcone derivatives from the fern Cyclosorus parasiticus and their anti-proliferative activity. Food Chem Toxicol. 2013; 60:147–152.
Article15. Glassmann A, Reichmann K, Scheffler B, Glas M, Veit N, Probstmeier R. Pharmacological targeting of the constitutively activated MEK/MAPK-dependent signaling pathway in glioma cells inhibits cell proliferation and migration. Int J Oncol. 2011; 39:1567–1575.
Article16. Jiao JW, Wen F. Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol Rep. 2011; 25:781–788.
Article17. Park IJ, Kim MJ, Park OJ, Park MG, Choe W, Kang I, et al. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010; 298:88–98.
Article18. Lee WY, Liu KW, Yeung JH. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett. 2009; 285:46–57.
Article19. Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009; 69:193–202.
Article20. Ge Y, Cheng R, Zhou Y, Shen J, Peng L, Xu X, et al. Cryptotanshinone induces cell cycle arrest and apoptosis of multidrug resistant human chronic myeloid leukemia cells by inhibiting the activity of eukaryotic initiation factor 4E. Mol Cell Biochem. 2012; 368:17–25.
Article21. Lu L, Li C, Li D, Wang Y, Zhou C, Shao W, et al. Cryptotanshinone inhibits human glioma cell proliferation by suppressing STAT3 signaling. Mol Cell Biochem. 2013; 381:273–282.
Article22. Zhou J, Xu XZ, Hu YR, Hu AR, Zhu CL, Gao GS. Cryptotanshinone induces inhibition of breast tumor growth by cytotoxic CD4+ T cells through the JAK2/STAT4/ perforin pathway. Asian Pac J Cancer Prev. 2014; 15:2439–2445.
Article23. Chen L, Wang HJ, Xie W, Yao Y, Zhang YS, Wang H. Cryptotanshinone inhibits lung tumorigenesis and induces apoptosis in cancer cells in vitro and in vivo. Mol Med Rep. 2014; 9:2447–2452.
Article24. Chen L, Zheng SZ, Sun ZG, Wang AY, Huang CH, Punchard NA, et al. Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother Pharmacol. 2011; 68:17–27.
Article25. Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003; 22:8590–8607.
Article26. Cheng CY, Su CC. Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol Med Rep. 2010; 3:645–650.
Article27. Nizamutdinova IT, Lee GW, Son KH, Jeon SJ, Kang SS, Kim YS, et al. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int J Oncol. 2008; 33:485–491.
Article28. Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007; 12:835–840.
Article29. Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011; 351:41–58.
Article30. Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014; 344:174–179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Amygdalin Regulates Apoptosis and Adhesion in Hs578T Triple-Negative Breast Cancer Cells
- Silibinin Enhances Ultraviolet B-Induced Apoptosis in MCF-7 Human Breast Cancer Cells
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways
- Effects of retinoic acid isomers on apoptosis and enzymatic antioxidant system in human breast cancer cells