J Breast Cancer.
2011 Mar;14(1):8-13. 10.4048/jbc.2011.14.1.8.
Silibinin Enhances Ultraviolet B-Induced Apoptosis in MCF-7 Human Breast Cancer Cells
- Affiliations
-
- 1Department of Biochemistry, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 2Division of Breast, Thyroid Surgery, Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea. shjung@jbnu.ac.kr
- KMID: 2175636
- DOI: http://doi.org/10.4048/jbc.2011.14.1.8
Abstract
- PURPOSE
Chemotherapies for breast cancer generally have strong cellular cytotoxicity and severe side effects. Thus, significant emphasis has been placed on combinations of naturally occurring chemopreventive agents. Silibinin is a major bioactive flavonolignan extracted from milk thistle with chemopreventive activity in various organs including the skin, prostate, and breast. However, the mechanism underlying the inhibitory action of silibinin in breast cancer has not been completely elucidated. Therefore, we investigated the effect of silibinin in MCF-7 human breast cancer cells and determined whether silibinin enhances ultraviolet (UV) B-induced apoptosis.
METHODS
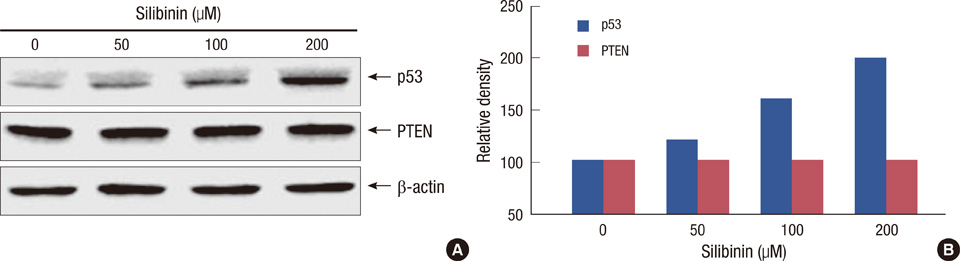
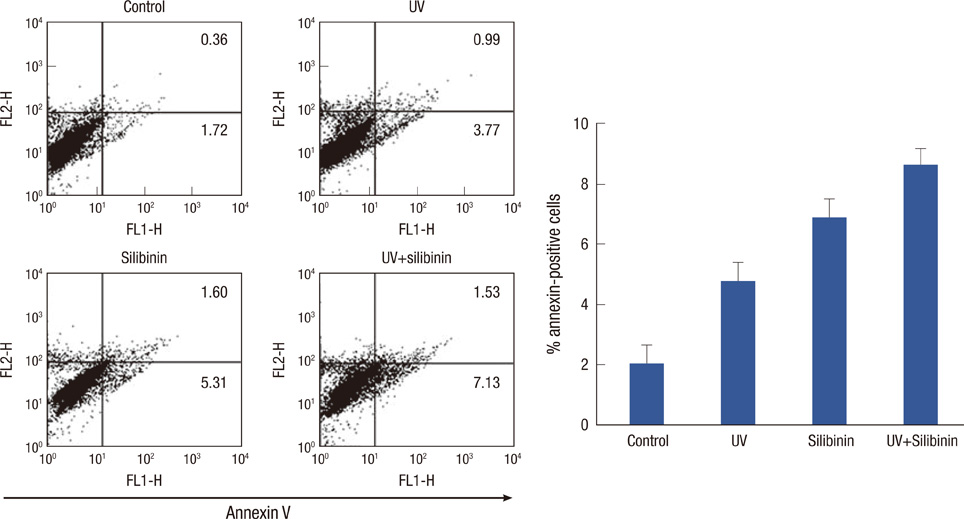
The effects of silibinin on MCF-7 cell viability were determined using the MTT assay. The effect of silibinin on PARP cleavage, as the hallmark of apoptotic cell death, and p53 protein expression in MCF-7 cells was analyzed using Western blot. The effect of silibinin on UVB-induced apoptosis in MCF-7 cells was analyzed by flow cytometry.
RESULTS
A dose- and time-dependent reduction in viability was observed in MCF-7 cells treated with silibinin. Silibinin strongly induced apoptotic cell death in MCF-7 cells, and induction of apoptosis was associated with increased p53 expression. Moreover, silibinin enhanced UVB-induced apoptosis in MCF-7 cells.
CONCLUSION
Silibinin induced a loss of cell viability and apoptotic cell death in MCF-7 cells. Furthermore, the combination of silibinin and UVB resulted in an additive effect on apoptosis in MCF-7 cells. These results suggest that silibinin might be an important supplemental agent for treating patients with breast cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Silibinin-Induced Apoptosis and Downregulation of MicroRNA-21 and MicroRNA-155 in MCF-7 Human Breast Cancer Cells
Masoud Maleki Zadeh, Nasrin Motamed, Najmeh Ranji, Mohammad Majidi, Fahimeh Falahi
J Breast Cancer. 2016;19(1):45-52. doi: 10.4048/jbc.2016.19.1.45.
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.
Article2. Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem Biophys Res Commun. 2007. 354:165–171.
Article3. Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB 468 cells. Oncol Rep. 2004. 11:493–499.4. Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008. 272:61–69.
Article5. Kaur M, Agarwal R. Silymarin and epithelial cancer chemoprevention: how close we are to bedside? Toxicol Appl Pharmacol. 2007. 224:350–359.
Article6. Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006. 45:436–442.
Article7. Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001. 15:465–489.
Article8. Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002. 62:3063–3069.9. Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997. 89:556–566.
Article10. Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, et al. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002. 101:461–468.
Article11. Flaig T, Agarwal R, Su L, Harrison GS, Gustafson DL, Glode LM. A phase I study of silibinin in hormone refractory prostate cancer. 2005. 23:In : 2005 ASCO Annual Meeting; Abstract #4698.12. Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009. 16:573–580.
Article13. Yarosh DB, Boumakis S, Brown AB, Canning MT, Galvin JW, Both DM, et al. Measurement of UVB-induced DNA damage and its consequences in models of immunosuppression. Methods. 2002. 28:55–62.
Article14. Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000. 16:195–201.
Article15. John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999. 8:399–406.16. Ferguson HA, Marietta PM, Van Den Berg CL. UV-induced apoptosis is mediated independent of caspase-9 in MCF-7 cells: a model for cytochrome c resistance. J Biol Chem. 2003. 278:45793–45800.17. Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004. 25:99–106.
Article18. Vogel G, Trost W, Braatz R, Odenthal KP, Brüsewitz G, Antweiler H, et al. Pharmacodynamics, site and mechanism of action of silymarin, the antihepatoxic principle from Silybum mar. (L) Gaertn. 1. Acute toxicology or tolerance, general and specific (liver-) pharmacology. Arzneimittelforschung. 1975. 25:82–89.19. Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991. 8:273–277.20. Katiyar SK, Roy AM, Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther. 2005. 4:207–216.21. Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 1999. 147:77–84.
Article22. Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991. 7:663–698.
Article23. Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993. 262:695–700.
Article24. Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000. 21:485–495.
Article25. Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989. 57:1083–1093.
Article26. Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005. 280:20375–20383.
Article27. Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005. 26:1404–1413.
Article28. Tonks NK, Myers MP. Structural assets of a tumor suppressor. Science. 1999. 286:2096–2097.
Article29. Mohan S, Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin modulates UVB-induced apoptosis via mitochondrial proteins, caspases activation, and mitogen-activated protein kinase signaling in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2004. 320:183–189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silibinin-Induced Apoptosis and Downregulation of MicroRNA-21 and MicroRNA-155 in MCF-7 Human Breast Cancer Cells
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Effects of retinoic acid isomers on apoptosis and enzymatic antioxidant system in human breast cancer cells
- The Role of bcl-2 and p53 in Tamoxifen-Induced Apoptosis of Human Breast Cancer Cell Lines
- Regulatory Role of Autophagy in Globular Adiponectin-Induced Apoptosis in Cancer Cells