Korean J Ophthalmol.
2016 Dec;30(6):468-478. 10.3341/kjo.2016.30.6.468.
Tunicamycin-induced Endoplasmic Reticulum Stress Upregulates the Expression of Pentraxin 3 in Human Retinal Pigment Epithelial Cells
- Affiliations
-
- 1School of Biological Sciences, College of Natural Sciences, University of Ulsan, Ulsan, Korea.
- 2Department of Ophthalmology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. imbus68@naver.com
- KMID: 2374005
- DOI: http://doi.org/10.3341/kjo.2016.30.6.468
Abstract
- PURPOSE
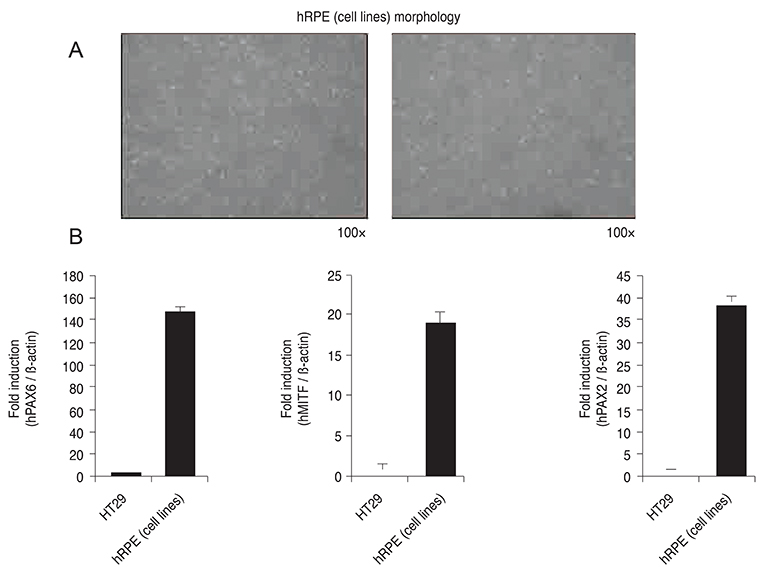
To investigate the production of long pentraxin 3 (PTX3) in response to tunicamycin-induced endoplasmic reticulum (ER) stress and its role in ER stress-associated cell death, PTX3 expression was evaluated in the human retinal pigment epithelial cell line, ARPE-19.
METHODS
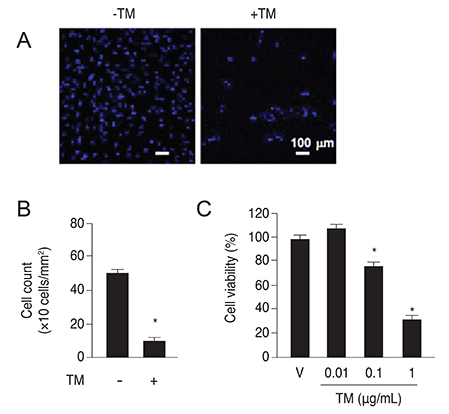
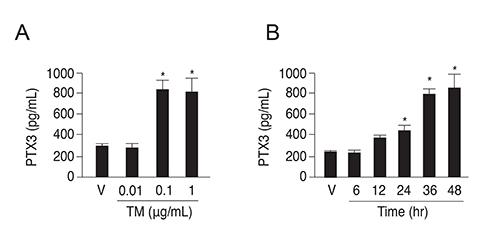
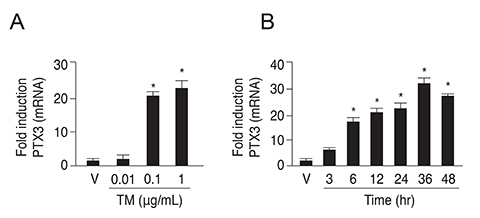
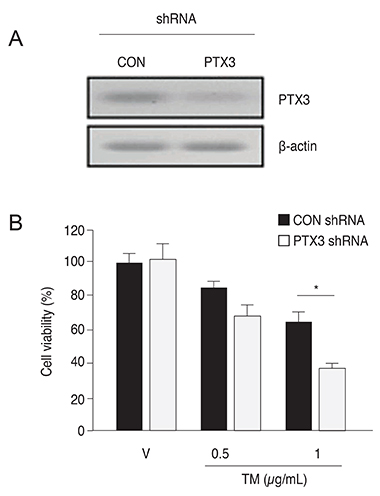
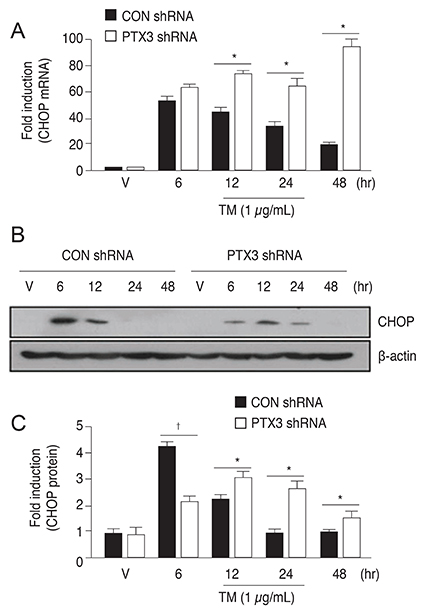
PTX3 production in ARPE-19 cells was analyzed in the absence or presence of tunicamycin treatment by enzyme-linked immunosorbent assay. PTX3 protein and mRNA levels were estimated using western blot analysis and real-time reverse transcription-polymerase chain reaction, respectively. Protein and mRNA levels of CCAAT-enhancer-binding protein homologous protein (CHOP) and ARPE-19 cell viability were measured in the presence of tunicamycin-induced ER stress in control or PTX3 small hairpin RNA (shRNA)-transfected ARPE-19 cells.
RESULTS
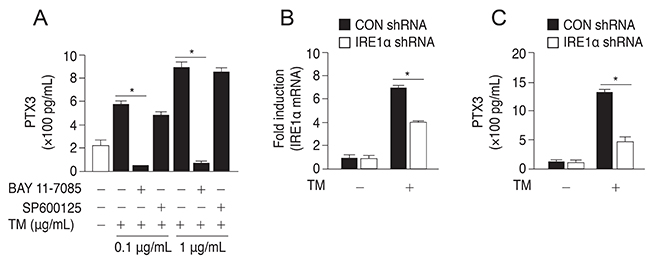
The protein and mRNA levels of PTX3 were found to be significantly increased by tunicamycin treatment. PTX3 production was significantly decreased in inositol-requiring enzyme 1α shRNA-transfected ARPE-19 cells compared to control shRNA-transfected cells. Furthermore, pretreatment with the NF-κB inhibitor abolished tunicamycin-induced PTX3 production. Decreased cell viability and prolonged protein and mRNA expression of CHOP were observed under tunicamycin-induced ER stress in PTX3 shRNA transfected ARPE-19 cells.
CONCLUSIONS
These results suggest that PTX3 production increased in the presence of tunicamycin-induced ER stress. Therefore, PTX3 could be an important protector of ER stress-induced cell death in human retinal pigment epithelial cells. Inositol-requiring enzyme 1α and the NF-κB signaling pathway may serve as potential targets for regulation of PTX3 expression in the retina. Therefore, their role in PTX3 expression needs to be further investigated.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/pharmacology
Apoptosis
Blotting, Western
C-Reactive Protein/biosynthesis/*genetics
Cells, Cultured
Endoplasmic Reticulum Stress/*drug effects/genetics
Enzyme-Linked Immunosorbent Assay
*Gene Expression Regulation
Humans
Polymerase Chain Reaction
RNA, Messenger/*genetics
Retinal Pigment Epithelium/*metabolism/pathology
Serum Amyloid P-Component/biosynthesis/*genetics
Tunicamycin/*pharmacology
Anti-Bacterial Agents
C-Reactive Protein
RNA, Messenger
Serum Amyloid P-Component
Tunicamycin
Figure
Reference
-
1. Goodman AR, Cardozo T, Abagyan R, et al. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996; 7:191–202.2. Hamel CP, Detrick B, Hooks JJ. Evaluation of Ia expression in rat ocular tissues following inoculation with interferon-gamma. Exp Eye Res. 1990; 50:173–182.3. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005; 23:337–366.4. Alles VV, Bottazzi B, Peri G, et al. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994; 84:3483–3493.5. Doni A, Peri G, Chieppa M, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003; 33:2886–2893.6. Luchetti MM, Piccinini G, Mantovani A, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin Exp Immunol. 2000; 119:196–202.7. Bussolati B, Peri G, Salvidio G, et al. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol. 2003; 170:1466–1472.8. Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002; 420:182–186.9. Garlanda C, Maina V, Cotena A, Moalli F. The soluble pattern recognition receptor pentraxin-3 in innate immunity, inflammation and fertility. J Reprod Immunol. 2009; 83:128–133.10. Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000; 96:4300–4306.11. van Rossum AP, Fazzini F, Limburg PC, et al. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004; 50:2667–2674.12. Jaillon S, Jeannin P, Hamon Y, et al. Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ. 2009; 16:465–474.13. Guo P, Zhang SZ, He H, et al. PTX3 controls activation of matrix metalloproteinase 1 and apoptosis in conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci. 2012; 53:3414–3423.14. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110:1389–1398.15. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.16. Okada T, Yoshida H, Akazawa R, et al. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002; 366:585–594.17. Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. 2003; 11:91–105.18. Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002; 42:487–495.19. Chung SW, Chen YH, Perrella MA. Role of Ets-2 in the regulation of heme oxygenase-1 by endotoxin. J Biol Chem. 2005; 280:4578–4584.20. Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010; 664:403–409.21. Salminen A, Kauppinen A, Hyttinen JM, et al. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010; 16:535–542.22. Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012; 2012:589589.23. Zhang SX, Sanders E, Wang JJ. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011; 4:51–61.24. Gardner BM, Pincus D, Gotthardt K, et al. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013; 5:a013169.25. Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by surfactant protein C BRICHOS mutants. Int J Biochem Cell Biol. 2012; 44:101–112.26. Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011; 91:1219–1243.27. Li J, Cai X, Xia Q, et al. Involvement of endoplasmic reticulum stress in all-trans-retinal-induced retinal pigment epithelium degeneration. Toxicol Sci. 2015; 143:196–208.28. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011; 13:184–190.29. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004; 11:381–389.30. Chen C, Cano M, Wang JJ, et al. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal. 2014; 20:2091–2106.31. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85:845–881.32. Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993; 17:189–195.33. Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008; 146:348–349.34. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705–732.35. Zou X, Feng Z, Li Y, et al. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem. 2012; 23:994–1006.36. Lim SK, Park MJ, Lim JC, et al. Hyperglycemia induces apoptosis via CB1 activation through the decrease of FAAH 1 in retinal pigment epithelial cells. J Cell Physiol. 2012; 227:569–577.37. Dou G, Sreekumar PG, Spee C, et al. Deficiency of αB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012; 53:1111–1122.38. Shimazawa M, Inokuchi Y, Ito Y, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007; 13:578–587.39. Chen L, Gao X. Neuronal apoptosis induced by endoplasmic reticulum stress. Neurochem Res. 2002; 27:891–898.40. Paschen W, Mengesdorf T, Althausen S, Hotop S. Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem. 2001; 76:1916–1924.41. Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999; 155:302–314.42. Kline CL, Schrufer TL, Jefferson LS, Kimball SR. Glucosamine-induced phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 is mediated by the protein kinase R-like endoplasmic-reticulum associated kinase. Int J Biochem Cell Biol. 2006; 38:1004–1014.43. Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005; 46:3973–3979.44. Breviario F, d'Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells: cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992; 267:22190–22197.45. Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor-and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993; 150:1804–1812.46. Napoleone E, Di Santo A, Bastone A, et al. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002; 22:782–787.47. Luchetti MM, Sambo P, Majlingova P, et al. Scleroderma fibroblasts constitutively express the long pentraxin PTX3. Clin Exp Rheumatol. 2004; 22:3 Suppl 33. S66–S72.48. May L, Kuningas M, van Bodegom D, et al. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol Reprod. 2010; 82:299–304.49. Bottazzi B, Bastone A, Doni A, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006; 79:909–912.50. Baruah P, Propato A, Dumitriu IE, et al. The pattern recognition receptor PTX3 is recruited at the synapse between dying and dendritic cells, and edits the cross-presentation of self, viral, and tumor antigens. Blood. 2006; 107:151–158.51. Baruah P, Dumitriu IE, Peri G, et al. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol. 2006; 80:87–95.52. Woo JM, Kwon MY, Shin DY, et al. Human retinal pigment epithelial cells express the long pentraxin PTX3. Mol Vis. 2013; 19:303–310.53. Min JK, Kim J, Woo JM. Elevated plasma pentraxin3 levels and its association with neovascular age-related macular degeneration. Ocul Immunol Inflamm. 2015; 23:205–211.54. Yang HS, Woo JE, Lee SJ, et al. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2014; 55:5989–5997.55. Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol. 2012; 26:562–573.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of Orai1 in tunicamycin-induced endothelial dysfunction
- Endoplasmic Reticulum Stress Induces MUC5AC and MUC5B Expression in Human Nasal Airway Epithelial Cells
- The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells
- Cynaropicrin Induces Reactive Oxygen Species-Dependent Paraptosis-Like Cell Death in Human Liver Cancer Cells
- Endoplasmic Reticulum Stress and Diabetes