Korean Diabetes J.
2010 Oct;34(5):312-319. 10.4093/kdj.2010.34.5.312.
The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. kgpark@dsmc.or.kr
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2029889
- DOI: http://doi.org/10.4093/kdj.2010.34.5.312
Abstract
- BACKGROUND
The highly developed endoplasmic reticulum (ER) structure in pancreatic beta cells is heavily involved in insulin biosynthesis. Thus, any perturbation in ER function inevitably impacts insulin biosynthesis. Recent studies showed that the expression of tribbles-related protein 3 (TRB3), a mammalian homolog of Drosophilia tribbles, in various cell types is induced by ER stress. Here, we examined whether ER stress induces TRB3 expression in INS-1 cells and found that TRB3 mediates ER stress-induced suppression of insulin gene expression.
METHODS
The effects of tunicamycin and thapsigargin on insulin and TRB3 expression in INS-1 cells were measured by Northern and Western blot analysis, respectively. The effects of adenovirus-mediated overexpression of TRB3 on insulin, PDX-1 and MafA gene expression in INS-1 cells were measured by Northern blot analysis. The effect of TRB3 on insulin promoter was measured by transient transfection study with constructs of human insulin promoter.
RESULTS
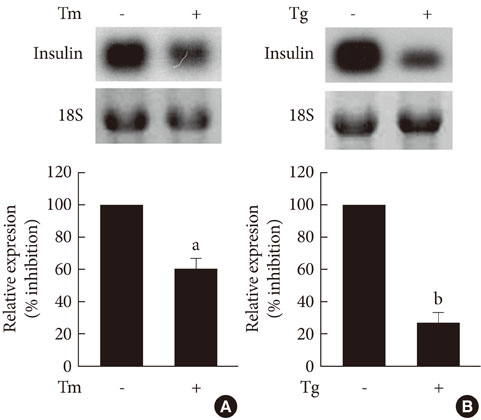
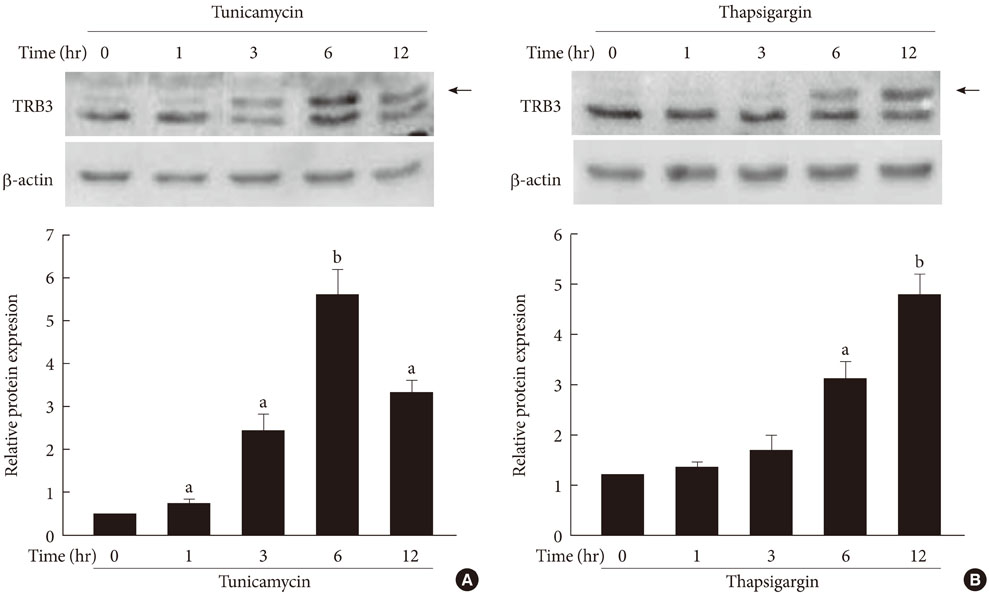
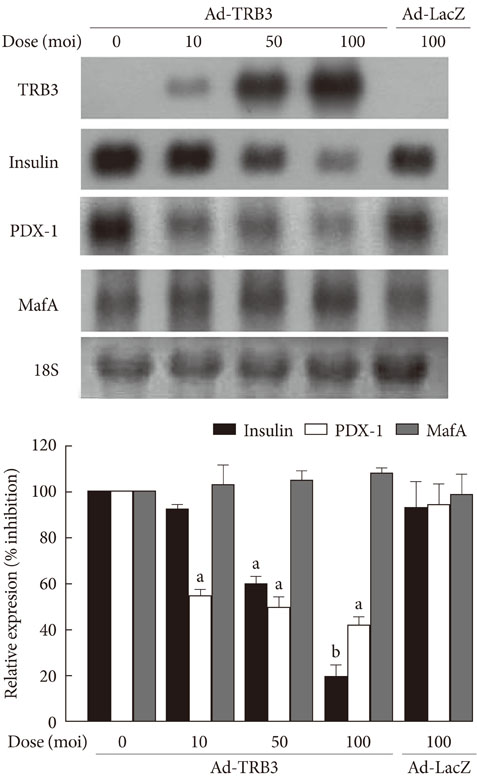
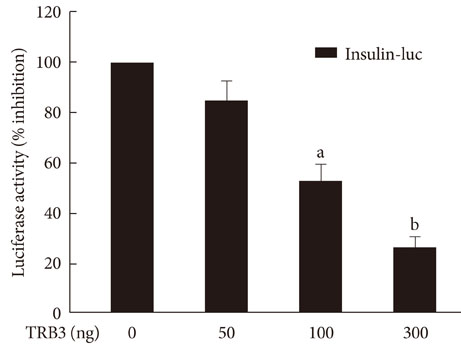
The treatment of INS-1 cells with tunicamycin and thapsigargin decreased insulin mRNA expression, but increased TRB3 protein expression. Adenovirus-mediated overexpression of TRB3 decreased insulin gene expression in a dose-dependent manner. A transient transfection study showed that TRB3 inhibited insulin promoter activity, suggesting that TRB3 inhibited insulin gene expression at transcriptional level. Adenovirus-mediated overexpression of TRB3 also decreased PDX-1 mRNA expression, but did not influence MafA mRNA expression.
CONCLUSIONS
This study showed that ER stress induced TRB3 expression, but decreased both insulin and PDX-1 gene expression in INS-1 cells. Our data suggest that TRB3 plays an important role in ER stress-induced beta cell dysfunction.
Keyword
MeSH Terms
Figure
Reference
-
1. Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002. 3:411–421.2. Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002. 7:335–345.3. Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000. 101:451–454.4. Becker KL. Principles and practice of endocrinology and metabolism. 2001. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;1296.5. Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998. 8:189–194.6. Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, Won KC, Park JY, Lee KU, Choi HS, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008. 149:3832–3841.7. Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002. 18:575–599.8. Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988. 332:462–464.9. Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998. 273:33741–33749.10. Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000. 101:249–258.11. Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood). 2003. 228:1213–1217.12. Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005. 24:1243–1255.13. Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003. 300:1574–1577.14. Okamoto H, Latres E, Liu R, Thabet K, Murphy A, Valenzeula D, Yancopoulos GD, Stitt TN, Glass DJ, Sleeman MW. Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis. Diabetes. 2007. 56:1350–1356.15. Selim E, Frkanec JT, Cunard R. Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression. Mol Immunol. 2007. 44:1218–1229.16. Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: novel regulators of cell function; evolutionary aspects. Cell Mol Life Sci. 2006. 63:1632–1641.17. Sung HY, Francis SE, Crossman DC, Kiss-Toth E. Regulation of expression and signalling modulator function of mammalian tribbles is cell-type specific. Immunol Lett. 2006. 104:171–177.18. Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. J Biol Chem. 2007. 282:24075–24082.19. Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity. Mol Cell Biol. 2007. 27:6818–6831.20. Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006. 312:1763–1766.21. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000. 6:1099–1108.22. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006. 4:245–254.23. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001. 7:1153–1163.24. Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005. 11:757–764.25. Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005. 54:1074–1081.26. Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007. 56:431–437.27. Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004. 10:530–534.28. Bi XP, Tan HW, Xing SS, Wang ZH, Tang MX, Zhang Y, Zhang W. Overexpression of TRB3 gene in adipose tissue of rats with high fructose-induced metabolic syndrome. Endocr J. 2008. 55:747–752.29. Corcoran CA, Luo X, He Q, Jiang C, Huang Y, Sheikh MS. Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Ther. 2005. 4:1063–1067.30. Qian B, Wang H, Men X, Zhang W, Cai H, Xu S, Xu Y, Ye L, Wollheim CB, Lou J. TRIB3 [corrected] is implicated in glucotoxicity-and endoplasmic reticulum-stress-induced [corrected] beta-cell apoptosis. J Endocrinol. 2008. 199:407–416.31. Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem. 2001. 276:25279–25286.32. Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002. 277:11225–11232.33. Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004. 114:828–836.34. Holland AM, Gonez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005. 54:2586–2595.35. Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005. 25:4969–4976.36. Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, Kojima I, Matsuhisa M, Hori M, Yamasaki Y. MafA regulates expression of genes important to islet beta-cell function. Mol Endocrinol. 2007. 21:2764–2774.37. Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994. 371:606–609.38. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998. 12:1763–1768.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Chronic High Glucose Concentration on Endoplasmic Reticulum Stress in INS-1 Cells
- Glucose Regulated Production of Human Insulin in Genetically Modified Myoblast Cell Line (C2C12)
- The Effects of Exendin-4 on IRS-2 Expression and Phosphorylation in INS-1 Cells
- Emodin exerts protective effect against palmitic acid-induced endoplasmic reticulum stress in HepG2 cells
- Microarray Analysis of Short Heterodimer Partner (SHP)-induced Changes in Gene Expression in INS-1 Cells