J Nutr Health.
2019 Apr;52(2):176-184. 10.4163/jnh.2019.52.2.176.
Emodin exerts protective effect against palmitic acid-induced endoplasmic reticulum stress in HepG2 cells
- Affiliations
-
- 1Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju, Jeonbuk 54896, Korea.
- 2Department of Food and Nutrition, Chungnam National University, Daejeon 34134, Korea. kakim@cnu.ac.kr
- KMID: 2444941
- DOI: http://doi.org/10.4163/jnh.2019.52.2.176
Abstract
- PURPOSE
Protein overloading in the endoplasmic reticulum (ER) leads to endoplasmic reticulum stress, which exacerbates various disease conditions. Emodin, an anthraquinone compound, is known to have several health benefits. The effect of emodin against palmitic acid (PA) - induced ER stress in HepG2 cells was investigated.
METHODS
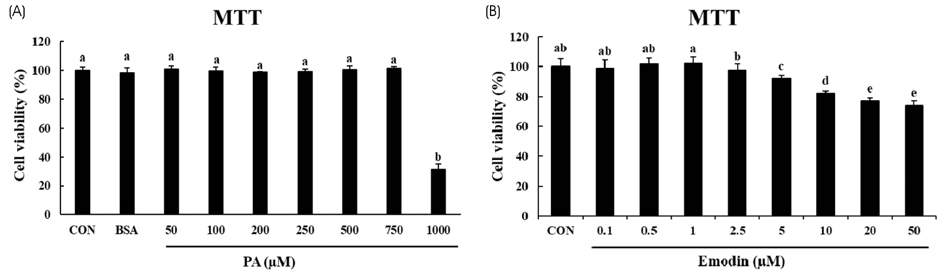
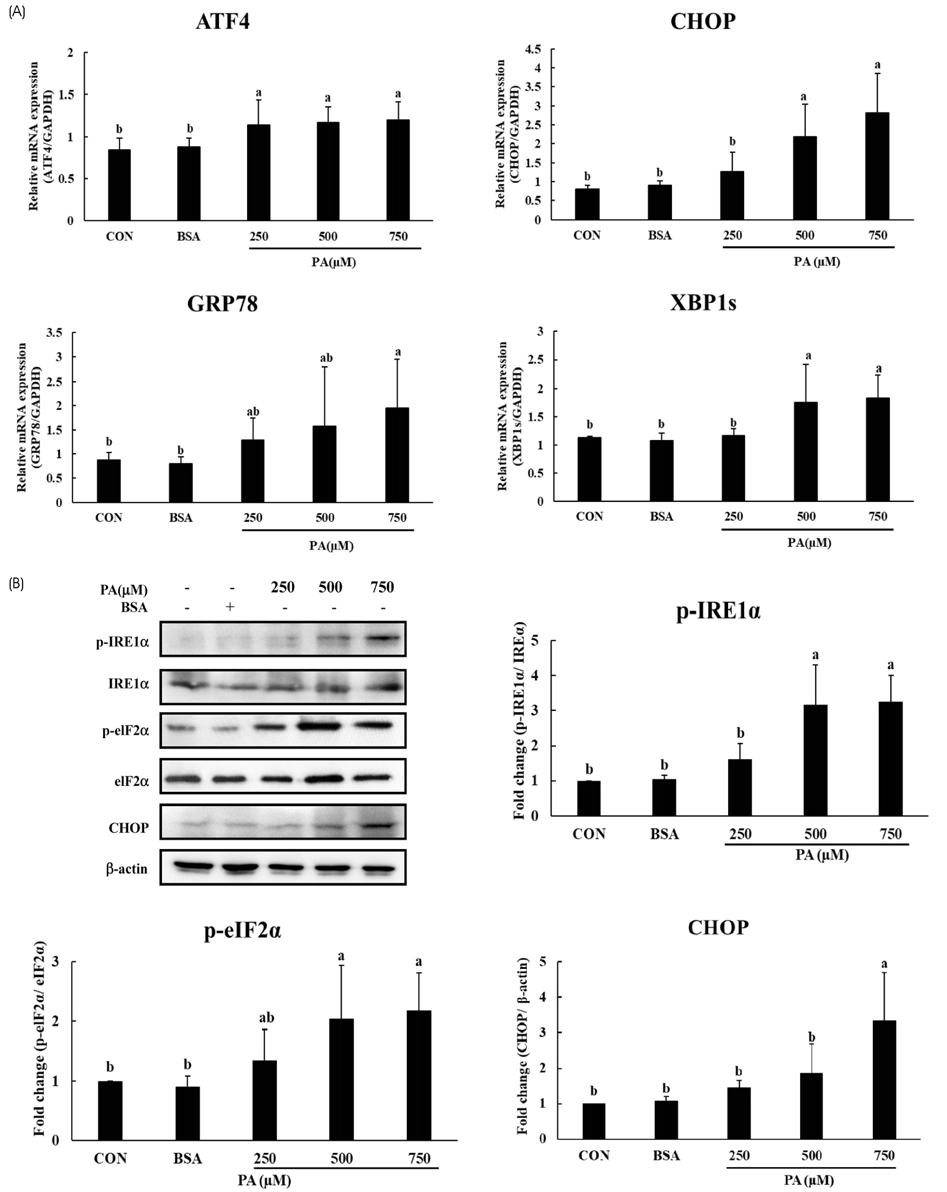
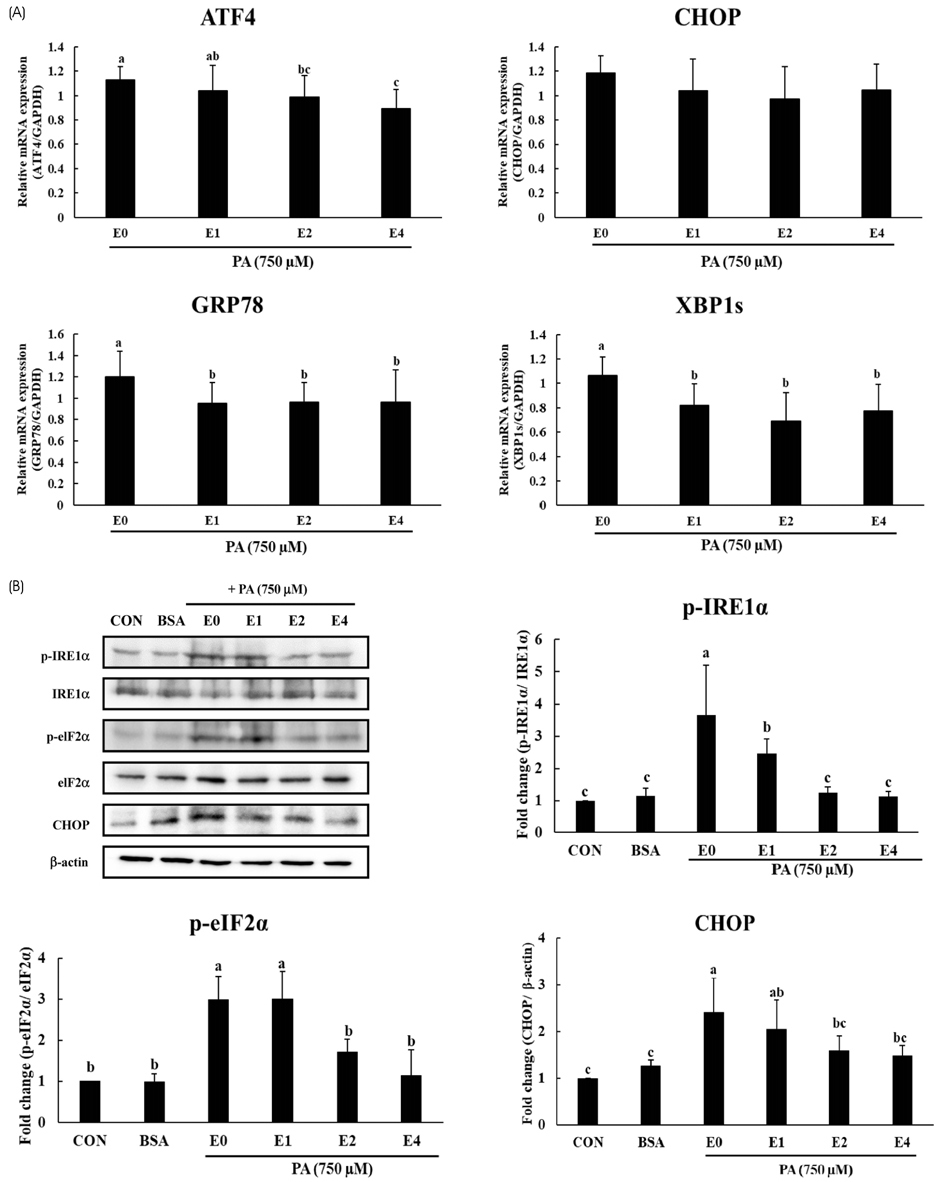
HepG2 cells were treated with varying concentrations of palmitic acid to determine the working concentration that induced ER stress. ER stress associated genes such as ATF4, XBP1s, CHOP and GRP78 were checked using RT- PCR. In addition, the expression levels of unfolded protein response (UPR) associated proteins such as IRE1α, eIF2α and CHOP were checked using immunoblotting to confirm the induction of ER stress. The effect of emodin on ER stress was analyzed by treating HepG2 cells with 750 µM palmitic acid and varying concentrations of emodin, then analyzing the expression of UPR associated genes.
RESULTS
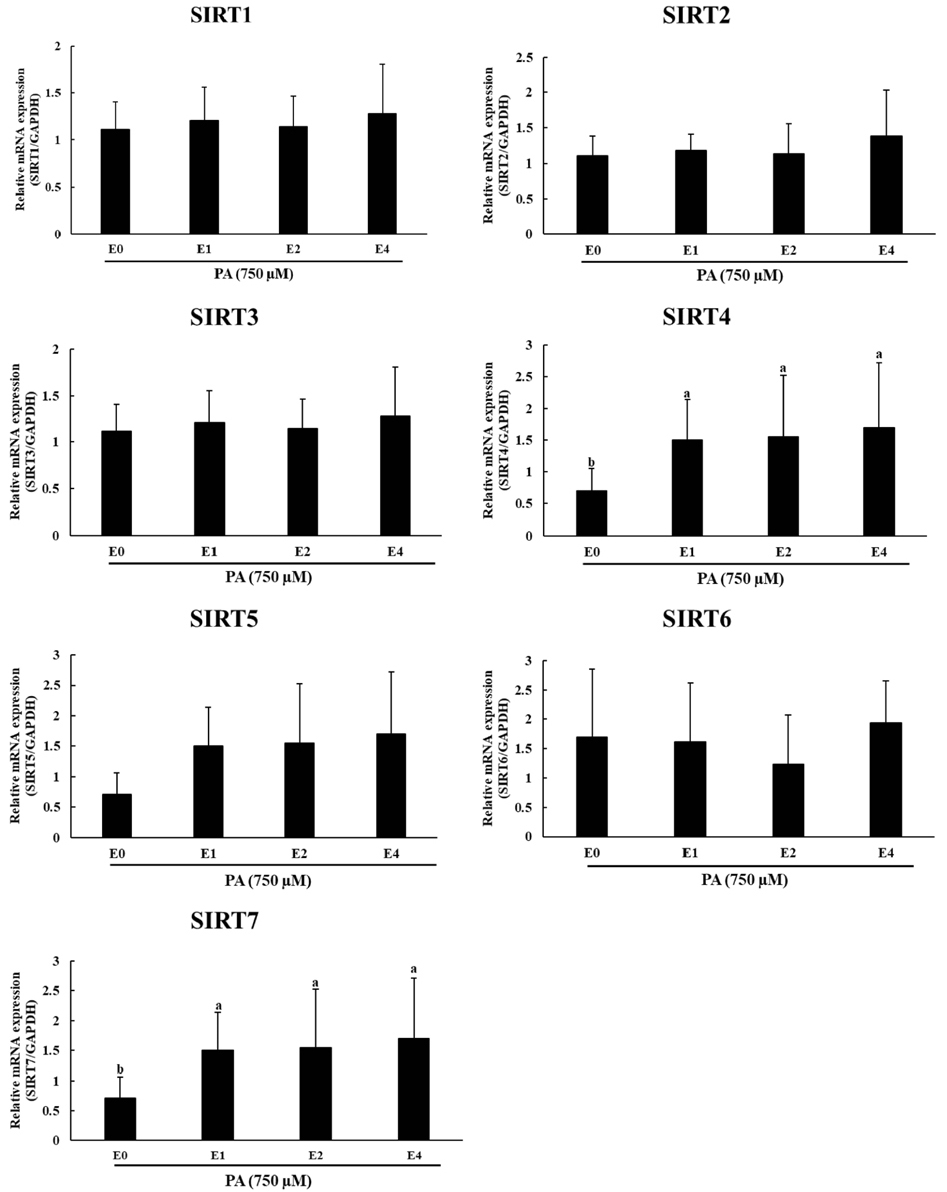
It was evident from the mRNA and protein expression results that palmitic acid significantly increased the expression of UPR associated genes and thereby induced ER stress. Subsequent treatment with emodin reduced the mRNA expression of ATF4, GRP78, and XBP1s. Furthermore, the protein levels of p-IRE1α, p-elF2α and CHOP were also reduced by the treatment of emodin. Analysis of sirtuin mRNA expression showed that emodin increased the levels of SIRT4 and SIRT7, indicating a possible role in decreasing the expression of UPR-related genes.
CONCLUSION
Altogether, the results suggest that emodin could exert a protective effect against fatty acid-induced ER stress and could be an agent for the management of various ER stress related diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006; 18(4):444–452.
Article2. Koga T, Suico MA, Shimasaki S, Watanabe E, Kai Y, Koyama K, et al. Endoplasmic reticulum (ER) stress induces Sirtuin 1 (SIRT1) expression via the PI3K-Akt-GSK3β signaling pathway and promotes hepatocellular injury. J Biol Chem. 2015; 290(51):30366–30374.
Article3. Graham RM, Hernandez F, Puerta N, De Angulo G, Webster KA, Vanni S. Resveratrol augments ER stress and the cytotoxic effects of glycolytic inhibition in neuroblastoma by downregulating Akt in a mechanism independent of SIRT1. Exp Mol Med. 2016; 48(2):e210.
Article4. Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005; 115(2):268–281.5. Miyagawa K, Oe S, Honma Y, Izumi H, Baba R, Harada M. Lipid-induced endoplasmic reticulum stress impairs selective autophagy at the step of autophagosome-lysosome fusion in hepatocytes. Am J Pathol. 2016; 186(7):1861–1873.
Article6. Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014; 8:213.
Article7. Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med. 2012; 18(10):589–598.
Article8. Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012; 11:1.
Article9. Katsoulieris E, Mabley JG, Samai M, Green IC, Chatterjee PK. α-Linolenic acid protects renal cells against palmitic acid lipotoxicity via inhibition of endoplasmic reticulum stress. Eur J Pharmacol. 2009; 623(1-3):107–112.
Article10. Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007; 293(2):E576–E586.
Article11. Dong X, Fu J, Yin X, Cao S, Li X, Lin L, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016; 30(8):1207–1218.
Article12. Wu L, Cai B, Liu X, Cai H. Emodin attenuates calcium overload and endoplasmic reticulum stress in AR42J rat pancreatic acinar cells. Mol Med Rep. 2014; 9(1):267–272.
Article13. Wu L, Cai B, Zheng S, Liu X, Cai H, Li H. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation. 2013; 36(5):1020–1029.
Article14. Kwak HJ, Choi HE, Cheon HG. 5-LO inhibition ameliorates palmitic acid-induced ER stress, oxidative stress and insulin resistance via AMPK activation in murine myotubes. Sci Rep. 2017; 7(1):5025.
Article15. Hwang SY, Heo K, Kim JS, Im JW, Lee SM, Cho M, et al. Emodin attenuates radioresistance induced by hypoxia in HepG2 cells via the enhancement of PARP1 cleavage and inhibition of JMJD2B. Oncol Rep. 2015; 33(4):1691–1698.
Article16. Dong X, Ni B, Fu J, Yin X, You L, Leng X, et al. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase-dependent pathway. Oncol Rep. 2018; 40(4):1985–1993.
Article17. Prola A, Pires Da Silva J, Guilbert A, Lecru L, Piquereau J, Ribeiro M, et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017; 24(2):343–356.
Article18. Zhang HH, Ma XJ, Wu LN, Zhao YY, Zhang PY, Zhang YH, et al. Sirtuin-3 (SIRT3) protects pancreatic β-cells from endoplasmic reticulum (ER) stress-induced apoptosis and dysfunction. Mol Cell Biochem. 2016; 420(1-2):95–106.
Article19. Huang HC, Tang D, Lu SY, Jiang ZF. Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer's disease. Neurol Res. 2015; 37(4):366–374.
Article20. Wang S, Binder P, Fang Q, Wang Z, Xiao W, Liu W, et al. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol. 2018; 175(8):1293–1304.
Article21. Yoshida H. ER stress and diseases. FEBS J. 2007; 274(3):630–658.
Article22. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.
Article23. Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013; 1833(12):3460–3470.
Article24. Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol. 2014; 307(7):C582–C594.25. Kiran S, Anwar T, Kiran M, Ramakrishna G. Sirtuin 7 in cell proliferation, stress and disease: Rise of the Seventh Sirtuin! Cell Signal. 2015; 27(3):673–682.
Article26. Paredes S, Villanova L, Chua KF. Molecular pathways: emerging roles of mammalian Sirtuin SIRT7 in cancer. Clin Cancer Res. 2014; 20(7):1741–1746.
Article27. Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, et al. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014; 19(5):649–656.
Article28. Yang T, Wang J, Pang Y, Dang X, Ren H, Liu Y, et al. Emodin suppresses silica-induced lung fibrosis by promoting Sirt1 signaling via direct contact. Mol Med Rep. 2016; 14(5):4643–4649.
Article29. Qu W, Wang Y, Wu Q, Liu J, Hao D. Emodin inhibits HMGB1-induced tumor angiogenesis in human osteosarcoma by regulating SIRT1. Int J Clin Exp Med. 2015; 8(9):15054–15064.30. Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Reports. 2013; 5(3):654–665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of Sargassum horneri extract against endoplasmic reticulum stress in HepG2 cells
- Exendin-4 Inhibits the Expression of SEPP1 and Fetuin-A via Improvement of Palmitic Acid-Induced Endoplasmic Reticulum Stress by AMPK
- Cynaropicrin Induces Reactive Oxygen Species-Dependent Paraptosis-Like Cell Death in Human Liver Cancer Cells
- Endoplasmic Reticulum Stress and Diabetes
- High-concentration Epigallocatechin Gallate Treatment Causes Endoplasmic Reticulum Stress-mediated Cell Death in HepG2 Cells