Endocrinol Metab.
2015 Jun;30(2):177-184. 10.3803/EnM.2015.30.2.177.
Exendin-4 Inhibits the Expression of SEPP1 and Fetuin-A via Improvement of Palmitic Acid-Induced Endoplasmic Reticulum Stress by AMPK
- Affiliations
-
- 1Institute of Medical Research, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. drlwy@hanmail.net
- KMID: 2282008
- DOI: http://doi.org/10.3803/EnM.2015.30.2.177
Abstract
- BACKGROUND
Selenoprotein P (SEPP1) and fetuin-A, both circulating liver-derived glycoproteins, are novel biomarkers for insulin resistance and nonalcoholic fatty liver disease. However, the effect of exendin-4 (Ex-4), a glucagon-like peptide-1 receptor agonist, on the expression of hepatokines, SEPP1, and fetuin-A, is unknown.
METHODS
The human hepatoma cell line HepG2 was treated with palmitic acid (PA; 0.4 mM) and tunicamycin (tuni; 2ug/ml) with or without exendin-4 (100 nM) for 24 hours. The change in expression of PA-induced SEPP1, fetuin-A, and endoplasmic reticulum (ER) stress markers by exendin-4 treatment were evaluated using quantitative real-time reverse transcription polymerase chain reaction and Western blotting. Transfection of cells with AMP-activated protein kinase (AMPK) small interfering RNA (siRNA) was performed to establish the effect of exendin-4-mediated AMPK in the regulation of SEPP1 and fetuin-A expression.
RESULTS
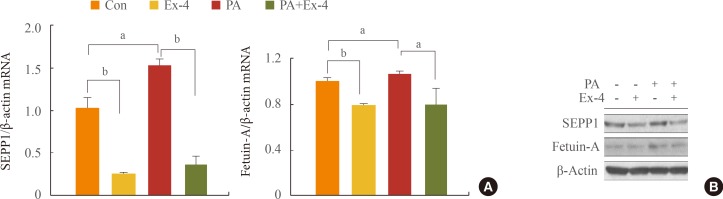
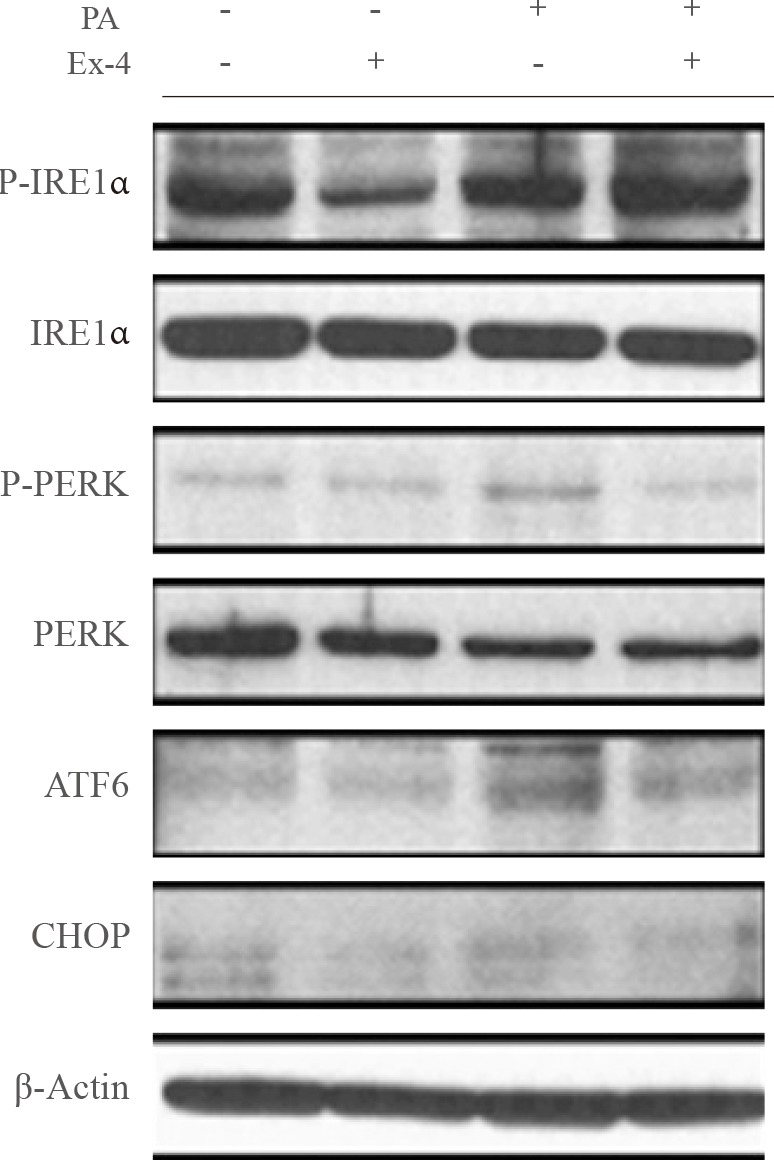
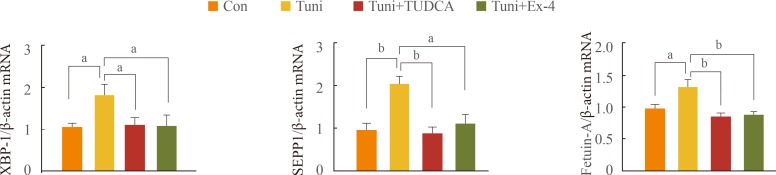
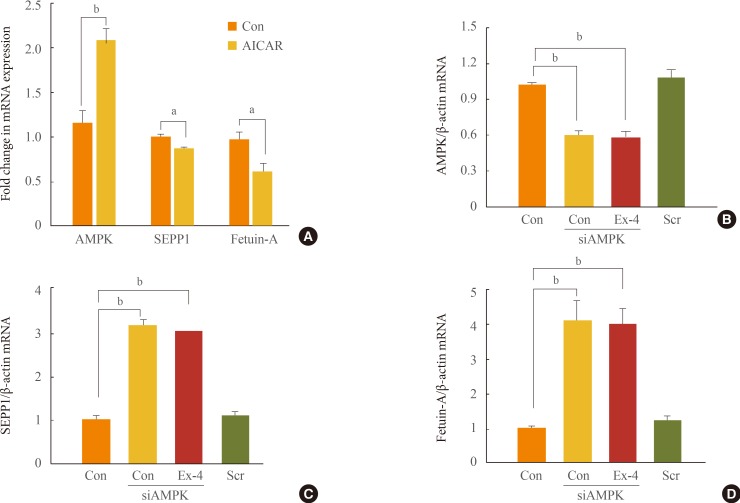
Exendin-4 reduced the expression of SEPP1, fetuin-A, and ER stress markers including PKR-like ER kinase, inositol-requiring kinase 1alpha, activating transcription factor 6, and C/EBP homologous protein in HepG2 cells. Exendin-4 also reduced the expression of SEPP1 and fetuin-A in cells treated with tunicamycin, an ER stress inducer. In cells treated with the AMPK activator 5-aminoidazole-4-carboxamide ribonucleotide (AICAR), the expression of hepatic SEPP1 and fetuin-A were negatively related by AMPK, which is the target of exendin-4. In addition, exendin-4 treatment did not decrease SEPP1 and fetuin-A expression in cells transfected with AMPK siRNA.
CONCLUSION
These data suggest that exendin-4 can attenuate the expression of hepatic SEPP1 and fetuin-A via improvement of PA-induced ER stress by AMPK.
Keyword
MeSH Terms
-
Activating Transcription Factor 6
alpha-2-HS-Glycoprotein*
AMP-Activated Protein Kinases*
Blotting, Western
Carcinoma, Hepatocellular
Cell Line
Endoplasmic Reticulum
Endoplasmic Reticulum Stress*
Fatty Liver
Glucagon-Like Peptide 1
Glycoproteins
Hep G2 Cells
Humans
Insulin Resistance
Palmitic Acid
Phosphotransferases
Polymerase Chain Reaction
Reverse Transcription
RNA, Small Interfering
Selenoprotein P
Transfection
Tunicamycin
Biomarkers
Glucagon-Like Peptide-1 Receptor
AMP-Activated Protein Kinases
Activating Transcription Factor 6
Glucagon-Like Peptide 1
Glycoproteins
Palmitic Acid
Phosphotransferases
RNA, Small Interfering
Selenoprotein P
Tunicamycin
alpha-2-HS-Glycoprotein
Figure
Cited by 2 articles
-
New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes
Won-Young Lee
Endocrinol Metab. 2017;32(1):1-5. doi: 10.3803/EnM.2017.32.1.1.Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
Inha Jung, Dae-Jeong Koo, Won-Young Lee
Diabetes Metab J. 2024;48(3):327-339. doi: 10.4093/dmj.2023.0350.
Reference
-
1. Walter PL, Steinbrenner H, Barthel A, Klotz LO. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008; 365:316–321. PMID: 17986386.
Article2. Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008; 48:1998–2006. PMID: 18972406.3. Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto K, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010; 12:483–495. PMID: 21035759.
Article4. Choi HY, Hwang SY, Lee CH, Hong HC, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Increased selenoprotein P levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab J. 2013; 37:63–71. PMID: 23439771.
Article5. Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011; 96:E1325–E1329. PMID: 21677040.
Article6. Misu H, Ishikura K, Kurita S, Takeshita Y, Ota T, Saito Y, Takahashi K, Kaneko S, Takamura T. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012; 7:e34952. PMID: 22496878.
Article7. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006; 29:853–857. PMID: 16567827.8. Haukeland JW, Dahl TB, Yndestad A, Gladhaug IP, Loberg EM, Haaland T, Konopski Z, Wium C, Aasheim ET, Johansen OE, Aukrust P, Halvorsen B, Birkeland KI. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol. 2012; 166:503–510. PMID: 22170794.
Article9. Zhao ZW, Lin CG, Wu LZ, Luo YK, Fan L, Dong XF, Zheng H. Serum fetuin-A levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes. Biomarkers. 2013; 18:160–164. PMID: 23410047.
Article10. Ou HY, Wu HT, Hung HC, Yang YC, Wu JS, Chang CJ. Endoplasmic reticulum stress induces the expression of fetuin-A to develop insulin resistance. Endocrinology. 2012; 153:2974–2984. PMID: 22619360.
Article11. Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, Grunberger G. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993; 7:1445–1455. PMID: 7906861.
Article12. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002; 51:2450–2458. PMID: 12145157.
Article13. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006; 43:173–181. PMID: 16374859.14. Lam S, See S. Exenatide: a novel incretin mimetic agent for treating type 2 diabetes mellitus. Cardiol Rev. 2006; 14:205–211. PMID: 16788334.15. Dorecka M, Siemianowicz K, Francuz T, Garczorz W, Chyra A, Klych A, Romaniuk W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol Rep. 2013; 65:884–890. PMID: 24145082.
Article16. Yang M, Zhang L, Wang C, Liu H, Boden G, Yang G, Li L. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012; 7:e48392. PMID: 23152772.
Article17. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006; 291:E275–E281. PMID: 16492686.
Article18. Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5’ monophosphate-activated protein kinase activation. Endocrinology. 2010; 151:576–585. PMID: 19952270.
Article19. Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Lee WY. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012; 7:e31394. PMID: 22363635.
Article20. Malin SK, Del Rincon JP, Huang H, Kirwan JP. Exercise-induced lowering of fetuin-A may increase hepatic insulin sensitivity. Med Sci Sports Exerc. 2014; 46:2085–2090. PMID: 24637346.
Article21. Ishibashi A, Ikeda Y, Ohguro T, Kumon Y, Yamanaka S, Takata H, Inoue M, Suehiro T, Terada Y. Serum fetuin-A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb. 2010; 17:925–933. PMID: 20543523.
Article22. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008; 3:e1765. PMID: 18335040.
Article23. Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008; 93:4479–4485. PMID: 18728159.
Article24. Mao J, Teng W. The relationship between selenoprotein P and glucose metabolism in experimental studies. Nutrients. 2013; 5:1937–1948. PMID: 23760059.
Article25. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009; 284:14809–14818. PMID: 19332540.
Article26. Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO. Associations of serum fetuin-A levels with insulin resistance and vascular complications in patients with type 2 diabetes. Diab Vasc Dis Res. 2013; 10:459–467. PMID: 23811603.
Article27. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306:457–461. PMID: 15486293.
Article28. Liu J, Jin X, Yu CH, Chen SH, Li WP, Li YM. Endoplasmic reticulum stress involved in the course of lipogenesis in fatty acids-induced hepatic steatosis. J Gastroenterol Hepatol. 2010; 25:613–618. PMID: 19929925.
Article29. Alhusaini S, McGee K, Schisano B, Harte A, McTernan P, Kumar S, Tripathi G. Lipopolysaccharide, high glucose and saturated fatty acids induce endoplasmic reticulum stress in cultured primary human adipocytes: salicylate alleviates this stress. Biochem Biophys Res Commun. 2010; 397:472–478. PMID: 20515657.
Article30. Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010; 107:1071–1082. PMID: 21030724.
Article31. Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005; 74:739–789. PMID: 15952902.32. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001; 107:881–891. PMID: 11779464.
Article33. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999; 339(Pt 1):135–141. PMID: 10085237.
Article34. Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014; 19:649–656. PMID: 24446069.
Article35. Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn BS, Baik SH, Choi KM. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein P through the AMPK-FOXO1α pathway. PLoS One. 2013; 8:e66529. PMID: 23825542.
Article36. Jung TW, Youn BS, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Kim BH, Baik SH, Choi KM. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013; 86:960–969. PMID: 23948064.
Article37. Takayama H, Misu H, Iwama H, Chikamoto K, Saito Y, Murao K, Teraguchi A, Lan F, Kikuchi A, Saito R, Tajima N, Shirasaki T, Matsugo S, Miyamoto K, Kaneko S, Takamura T. Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J Biol Chem. 2014; 289:335–345. PMID: 24257750.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emodin exerts protective effect against palmitic acid-induced endoplasmic reticulum stress in HepG2 cells
- Endoplasmic Reticulum Stress and Diabetes
- Inhibitory effects of Sargassum horneri extract against endoplasmic reticulum stress in HepG2 cells
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- Endoplasmic Reticulum Stress-Mediated p62 Downregulation Inhibits Apoptosis via c-Jun Upregulation