Korean Diabetes J.
2008 Apr;32(2):102-111. 10.4093/kdj.2008.32.2.102.
The Effects of Exendin-4 on IRS-2 Expression and Phosphorylation in INS-1 Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, The Catholic University of Korea, Korea.
- KMID: 2222506
- DOI: http://doi.org/10.4093/kdj.2008.32.2.102
Abstract
-
BACKGROUND: Insulin receptor substrate 2 (IRS-2) is a key regulator of beta cell proliferation and apoptosis. This study was aimed to investigate effect of the glucolipotoxicity on apoptosis in INS-1 cell, and the effect of Exendin-4, a GLP-1 receptor agonist, on IRS-2 expression in the glucolipotoxicity induced INS-1 cell. The goal was to discover the new action mechanism and function of Exendin-4 in beta cell apoptosis.
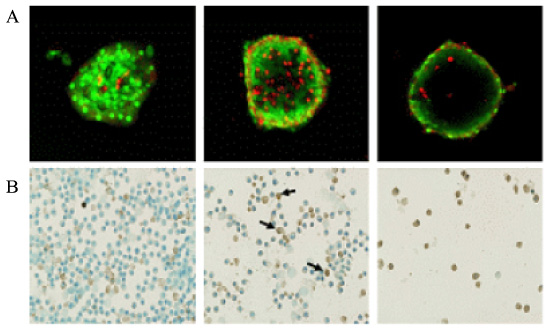
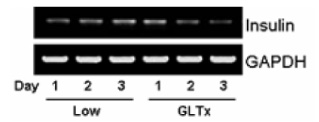
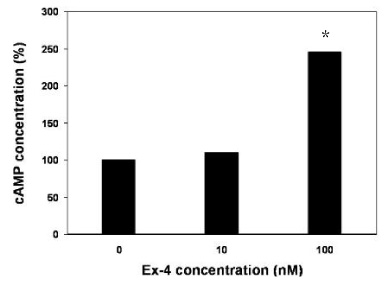
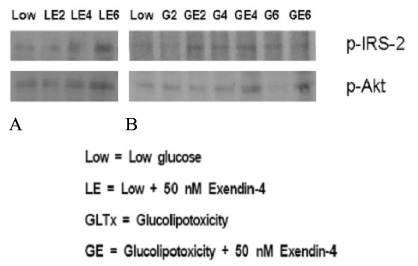
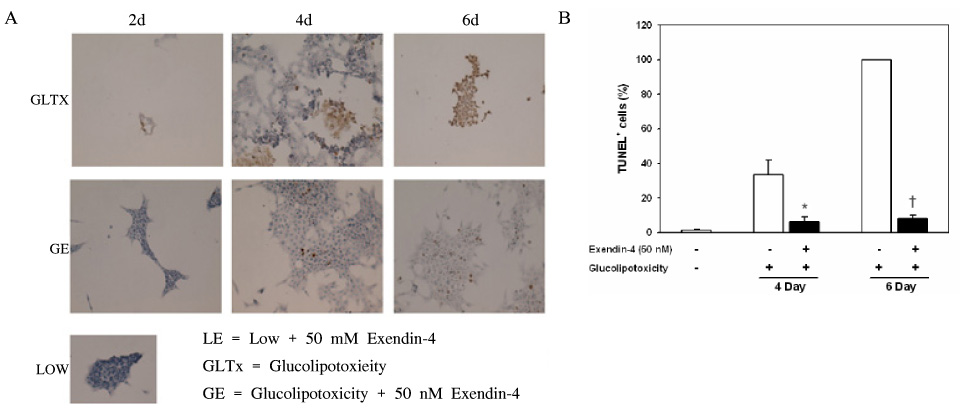
METHOD: INS-1 cells were cultured in glucolipotoxic condition for 2, 4 or 6 days and were categorized as G groups. Another group in which 50 nM Exendin-4 was added to INS-1 cells, cultured in glucolipotoxic condition, were named as Ex-4 groups. We investigated the expression of IRS-2 by RT-PCR, phosphorylated IRS-2 and phosphorylated Akt protein levels by western blot. We measured the apoptosis ratio of INS-1 cell in glucolipotoxic condition by TUNEL staining in both groups.
RESULT: IRS-2 expression of INS-1 cells decreased with correlation to the time of exposure to glucolipotoxic condition. pIRS-2 and pAkt protein levels decreased in the similar pattern in glucolipotoxicity group. However, this effect of glucolipotoxicity on INS-1 cell was inhibited by the Exendin-4 treatment. In the Ex-4 groups, IRS-2 expression, pIRS-2 and pAkt protein levels remained at the similar level to low glucose condition state. Also, apoptosis induced by glucolipotoxicity was suppressed by Exendin-4 treatment significantly.
CONCLUSION
We showed that the long-term treatment of Exendin-4 inhibited the apoptosis of beta cells significantly in glucolipotoxic condition and that this effect of Exendin-4 was related with IRS-2 and Akt among the beta cell's intracellular signal transduction pathway.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Cell Proliferation
Cells, Cultured
Glucagon-Like Peptide 1
Glucagon-Like Peptide-1 Receptor
Glucose
In Situ Nick-End Labeling
Insulin Receptor Substrate Proteins
Peptides
Phosphorylation
Receptors, Glucagon
Signal Transduction
Venoms
Glucagon-Like Peptide 1
Glucose
Insulin Receptor Substrate Proteins
Peptides
Receptors, Glucagon
Venoms
Figure
Reference
-
1. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes. principles of pathogenesis and therapy. Lancet. 2005. 365:1333–1346.
Article2. Reaven G, Tsao PS. Insulin resistance and compensatory hyperinsulinemia: the key player between cigarette smoking and cardiovascular disease? J Am Coll Cardiol. 2003. 41:1044–1047.3. Holman RR. Assessing the potential for α-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998. 40:Suppl. S21–S25.
Article4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.5. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998. 352:854–865.6. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003. 52:102–110.
Article7. Wajchenberg BL. β-Cell Failure in Diabetes and Preservation by Clinical Treatment. Endocrine Reviews. 2007. 28:187–218.
Article8. Creutzfeldt W. The incretin concept today. Diabetologia. 1979. 16:75–85.
Article9. Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000. 49:741–748.
Article10. Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002. 283:E745–E752.11. Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000. 141:4600–4605.
Article12. De Fronzo RA, Ratner RE, Han J, Kim D, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005. 28:1092–1100.
Article13. Giannoukakis N. Exenatide. Amylin/Eli Lilly. Curr Opin Investig Drus. 2003. 4:459–465.14. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003. 26:929–940.
Article15. Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White MF, Montminy M. cAMP promotes pancreatic-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003. 17:1575–1580.16. Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF. Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J Clin Invest. 2003. 112:1521–1532.
Article17. Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001. 56:377–399.
Article18. Kjems LL, Holst JJ, Volund A, Madsbad S. The Influence of GLP-1 on Glucose-Stimulated Insulin Secretion Effects on β-Cell Sensitivity in Type 2 and Nondiabetic Subjects. Diabetes. 2003. 52:380–386.
Article19. Holst JJ. Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab. Res Rev. 2002. 18:430–441.
Article20. Habener JF, Kemp DM. LeRoith D, Taylon SI, Olefsky JM, editors. Diabetes Mellitus. A Fundamental and Clinical Text. 2004. 3rd Ed. Philadelphia: Lippincott Williams & Wilkins;99–113.21. Chepurny OG, Hussain MA, Holz GG. Exendin-4 as a Stimulator of Rat Insulin I Gene Promoter Activity via bZIP/CRE Interactions Sensitive to Serine/Threonine Protein Kinase Inhibitor Ro 31-8220. Endocrinology. 2002. 143:2303–2313.
Article22. Holz GG. Epac: A New cAMP-Binding Protein in Support of Glucagon-Like Peptide-1 Receptor-Mediated Signal Transduction in the Pancreatic β-Cell. Diabetes. 2004. 53:5–13.
Article23. Park SM, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF. Exendin-4 Uses Irs2 Signaling to Mediate Pancreatic Cell Growth and Function. J Biol Chem. 2006. 281:1159–1168.24. Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein Kinase C Activation Mediates Glucagon-Like Peptide-1-Induced Pancreatic β-Cell Proliferation. Diabetes. 2001. 50:2237–2243.
Article25. Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic-cell growth and survival by the serine/threonine protein kinase Akt1/PKB. Nat Med. 2001. 7:1133–1137.26. Buteau J, El Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004. 47:806–815.
Article27. Lawlor MA, Alessi DR. PKB/Akt, a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001. 114:2903–2910.28. Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest. 2002. 110:1839–1847.
Article29. Roche E, Maestre I, Martin F, Fuentes E, Casero J, Reig JA, Soria B. Nutrient toxicity in pancreatic beta-cell dysfunction. J Physiol Biochem. 2000. 56:119–128.30. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.
Article31. Rossetti L, Giaccari A, De Fronzo RA. Glucose toxicity. Diabetes Care. 1990. 13:610–630.
Article32. Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003. 13:1575–1580.33. Giorgino F, Laviola L, Leonardini A, Natalicchio A. GLP-1: a new approach for type 2 diabetes therapy. Diabetes Res Clin Pract. 2006. 74:S152–S155.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of cAMP/PKA Activation on Exendin-4-Induced Cyclin D1 Expression in INS-1 Cell
- Telmisartan increases hepatic glucose production via protein kinase C ζ-dependent insulin receptor substrate-1 phosphorylation in HepG2 cells and mouse liver
- Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
- The Effect of Chronic High Glucose Concentration on Endoplasmic Reticulum Stress in INS-1 Cells
- Mitogenic Effects and Signaling Pathway of Insulin-Like Growth Factor-I (IGF-I) in the Rat Beta Cell Line (INS-1)