Korean J Physiol Pharmacol.
2019 Mar;23(2):95-102. 10.4196/kjpp.2019.23.2.95.
Involvement of Orai1 in tunicamycin-induced endothelial dysfunction
- Affiliations
-
- 1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Clinical Pharmacology, Guangzhou, Guangdong 510080, China. chunyudeng@126.com, raofang@medmail.com.cn
- 2Research Center of Medical Sciences, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong 510080, China.
- 3Department of Anesthesiology, Guangzhou Women and Children's Medical Center, Guangzhou, Guangdong 510623, China.
- 4Department of Pharmacy, Guangzhou Panyu Shiqiao Hospital, Guangzhou, Guangdong 511400, China.
- KMID: 2438083
- DOI: http://doi.org/10.4196/kjpp.2019.23.2.95
Abstract
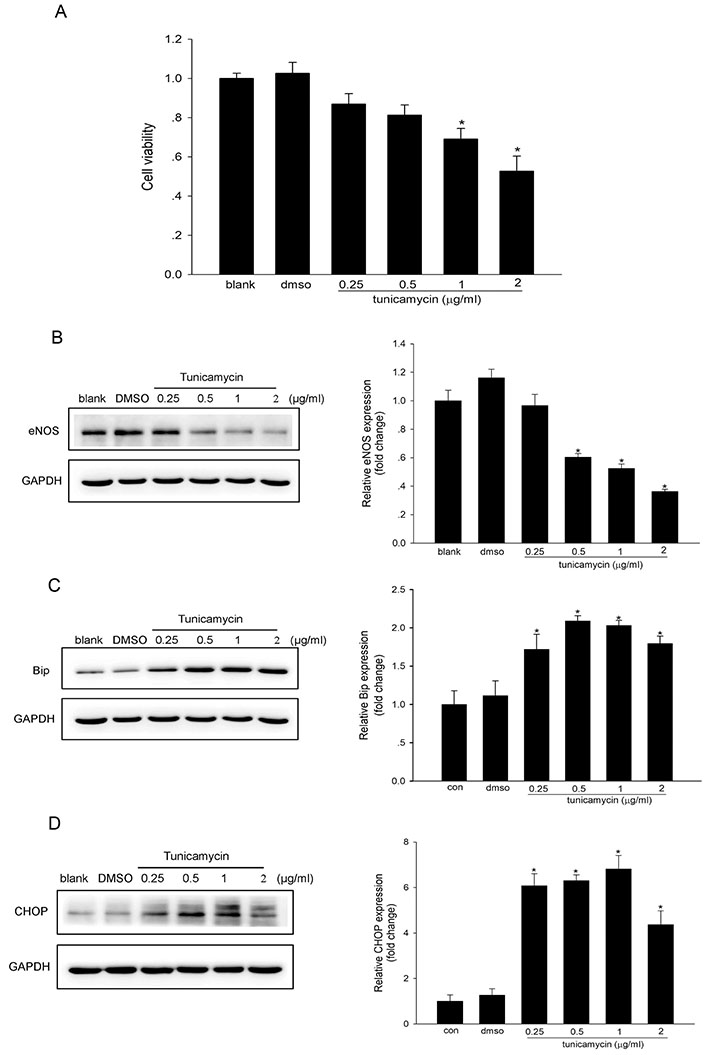
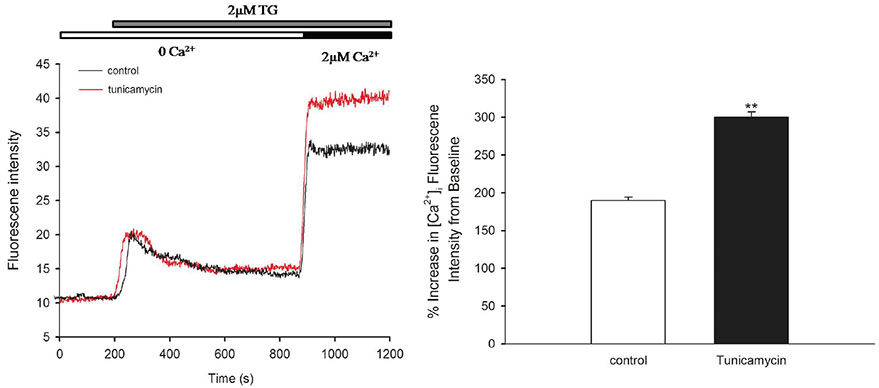
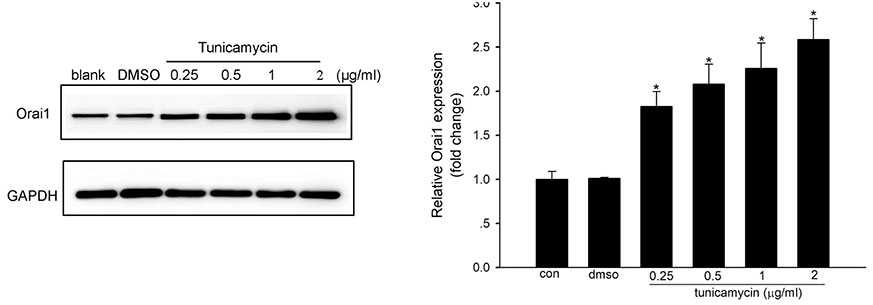
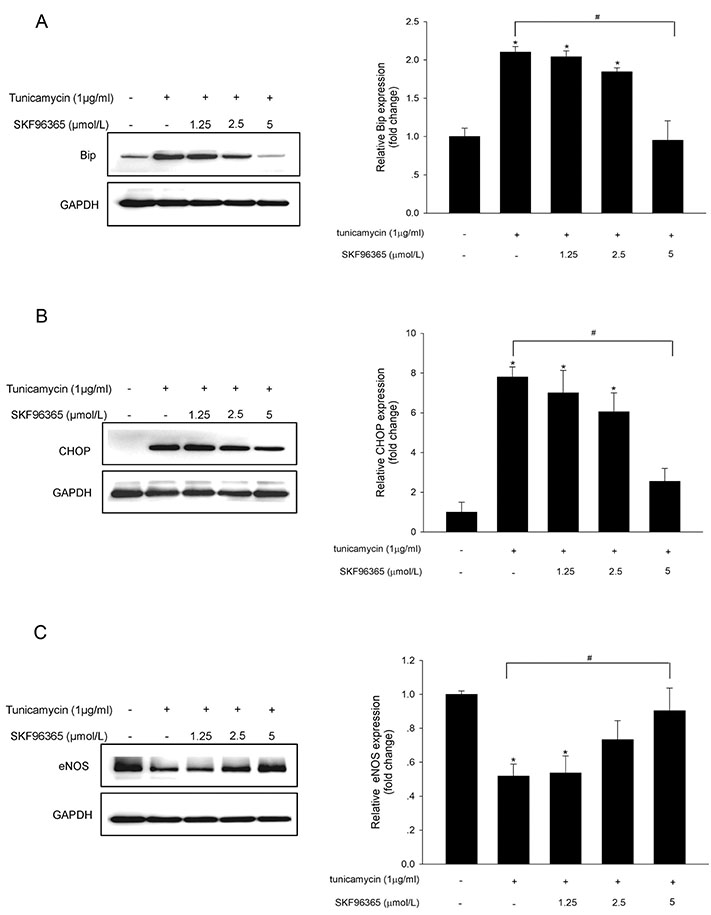
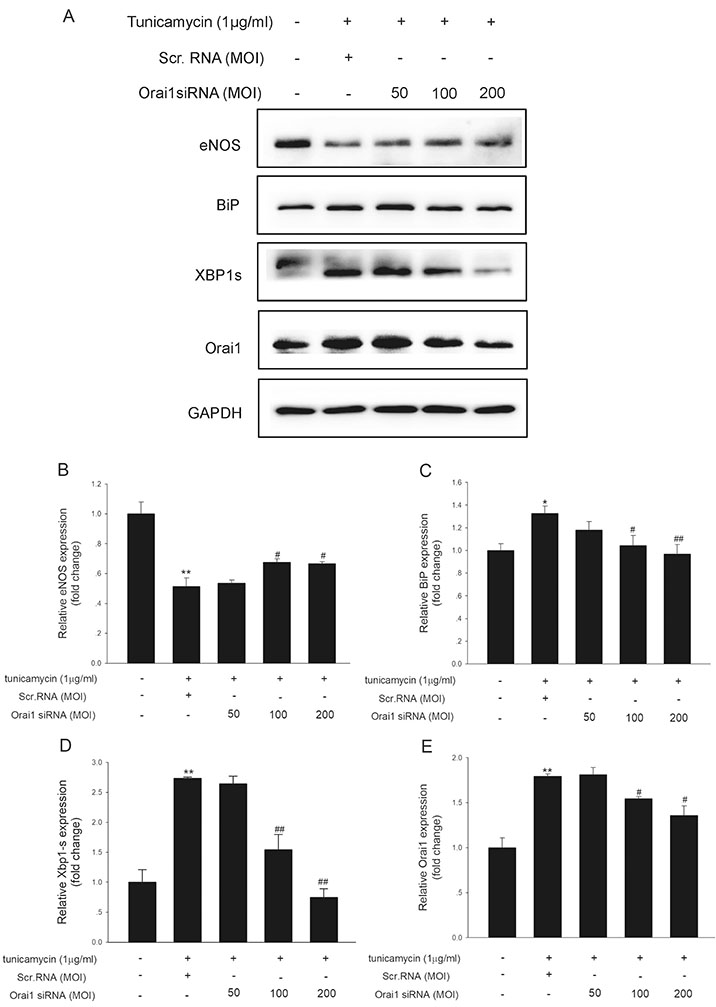
- Endoplasmic reticulum (ER) stress is mediated by disturbance of Ca²âº homeostasis. The store-operated calcium (SOC) channel is the primary Ca²âº channel in non-excitable cells, but its participation in agent-induced ER stress is not clear. In this study, the effects of tunicamycin on Ca²âº influx in human umbilical vein endothelial cells (HUVECs) were observed with the fluorescent probe Fluo-4 AM. The effect of tunicamycin on the expression of the unfolded protein response (UPR)-related proteins BiP and CHOP was assayed by western blotting with or without inhibition of Orai1. Tunicamycin induced endothelial dysfunction by activating ER stress. Orai1 expression and the influx of extracellular Ca²âº in HUVECs were both upregulated during ER stress. The SOC channel inhibitor SKF96365 reversed tunicamycin-induced endothelial cell dysfunction by inhibiting ER stress. Regulation of tunicamycin-induced ER stress by Orai1 indicates that modification of Orai1 activity may have therapeutic value for conditions with ER stress-induced endothelial dysfunction.
MeSH Terms
Figure
Reference
-
1. Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009; 120:1266–1286.
Article2. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003; 23:168–175.3. Lu Y, Qian L, Zhang Q, Chen B, Gui L, Huang D, Chen G, Chen L. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013; 92:1165–1173.
Article4. Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006; 26:2490–2496.
Article5. Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life. 2014; 66:530–537.
Article6. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.
Article7. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012; 81:767–793.
Article8. Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013; 75:49–67.
Article9. Krebs J, Agellon LB, Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem Biophys Res Commun. 2015; 460:114–121.10. Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annu Rev Physiol. 1997; 59:145–170.
Article11. Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and storeoperated calcium entry important for endothelial cell proliferation. Circ Res. 2008; 103:1289–1299.
Article12. Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007; 2:481–485.
Article13. Groenendyk J, Peng Z, Dudek E, Fan X, Mizianty MJ, Dufey E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y, Kim DH, Baretta K, Srikanth S, Gwack Y, Ahnn J, Kaufman RJ, Lee SK, Hetz C, Kurgan L, Michalak M. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal. 2014; 7:ra54.
Article14. Chen S, Zhang Z, Wu Y, Shi Q, Yan H, Mei N, Tolleson WH, Guo L. Endoplasmic reticulum stress and store-operated calcium entry contribute to usnic acid-induced toxicity in hepatic cells. Toxicol Sci. 2015; 146:116–126.
Article15. Czyź A, Brutkowski W, Fronk J, Duszyński J, Zabłocki K. Tunicamycin desensitizes store-operated Ca2+ entry to ATP and mitochondrial potential. Biochem Biophys Res Commun. 2009; 381:176–180.16. Ziomek G, Cheraghi Zanjani P, Arman D, van Breemen C, Esfandiarei M. Calcium regulation in aortic smooth muscle cells during the initial phase of tunicamycin-induced endo/sarcoplasmic reticulum stress. Eur J Pharmacol. 2014; 735:86–96.
Article17. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005; 15:1235–1241.18. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006; 443:230–233.
Article19. Zhou MH, Zheng H, Si H, Jin Y, Peng JM, He L, Zhou Y, Muñoz-Garay C, Zawieja DC, Kuo L, Peng X, Zhang SL. Stromal interaction molecule 1 (STIM1) and Orai1 mediate histamine-evoked calcium entry and nuclear factor of activated T-cells (NFAT) signaling in human umbilical vein endothelial cells. J Biol Chem. 2014; 289:29446–29456.
Article20. Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, Stacey M, O'Regan D, Foster R, Porter KE, Kearney MT, Beech DJ. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011; 108:1190–1198.21. Huang H, Jing G, Wang JJ, Sheibani N, Zhang SX. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm (Lond). 2015; 12:31.
Article22. Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor δ pathway. Arterioscler Thromb Vasc Biol. 2014; 34:830–836.
Article23. Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003; 5:781–792.
Article24. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005; 115:2656–2664.
Article25. Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004; 110:705–712.26. Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012; 122:1354–1367.
Article27. Stalker TJ, Gong Y, Scalia R. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005; 54:1132–1140.
Article28. Loinard C, Zouggari Y, Rueda P, Ramkhelawon B, Cochain C, Vilar J, Récalde A, Richart A, Charue D, Duriez M, Mori M, Arenzana-Seisdedos F, Lévy BI, Heymes C, Silvestre JS. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation. 2012; 125:1014–1026.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tunicamycin enhances TRAIL-induced apoptosis by inhibition of cyclin D1 and the subsequent downregulation of survivin
- Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes
- Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis
- Tunicamycin-induced Endoplasmic Reticulum Stress Upregulates the Expression of Pentraxin 3 in Human Retinal Pigment Epithelial Cells
- Lyso-globotriaosylsphingosine induces endothelial dysfunction via autophagy-dependent regulation of necroptosis