Korean J Physiol Pharmacol.
2023 May;27(3):231-240. 10.4196/kjpp.2023.27.3.231.
Lyso-globotriaosylsphingosine induces endothelial dysfunction via autophagy-dependent regulation of necroptosis
- Affiliations
-
- 1Department of Pharmacology, Yeungnam University College of Medicine, Daegu 42415, Korea
- 2Department of Physiology, Ewha Womans University College of Medicine, Seoul 07084, Korea
- KMID: 2541635
- DOI: http://doi.org/10.4196/kjpp.2023.27.3.231
Abstract
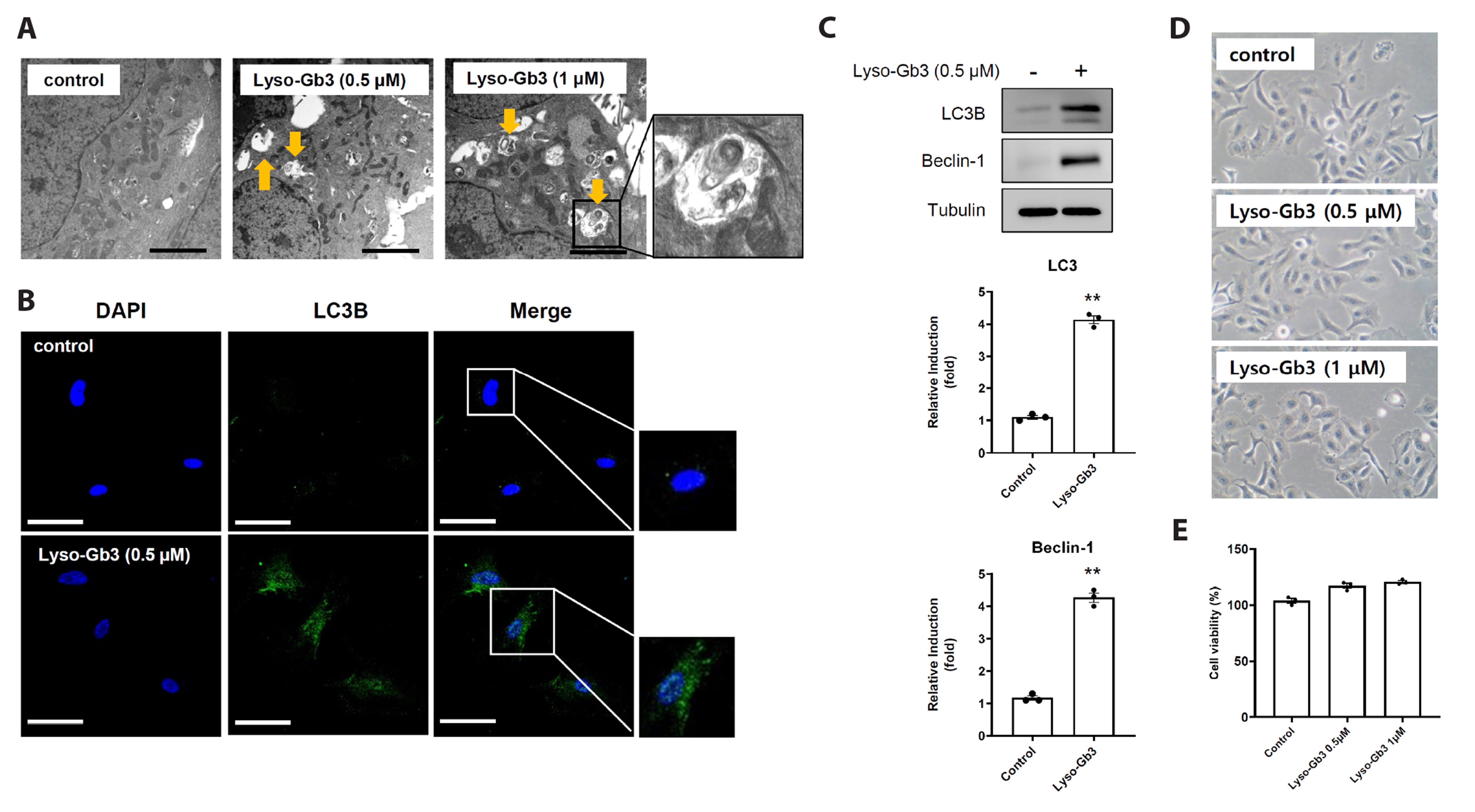
- Fabry disease is a lysosomal storage disorder characterized by the lysosomal accumulations of glycosphingolipids in a variety of cytotypes, which include endothelial cells. The disease is inherited and originates from an error in glycosphingolipid catabolism caused by insufficient α-galactosidase A activity, which causes uncontrolled progressive storage of intracellular globotriaosylceramide (Gb3) in the vasculature and extracellular accumulation of lyso-Gb3 (a deacetylated soluble form of Gb3). Necrosis can lead to inflammation, which exacerbates necrosis and creates a positive feedback loop that triggers necroinflammation. However, the role played by necroptosis, a form of programmed necrotic cell death, in the cell-to-cell inflammatory reaction between epithelial and endothelial cells is unclear. Thus, the present study was undertaken to determine whether lyso-Gb3 induces necroptosis and whether necroptosis inhibition protects endothelial dysfunction against lyso-Gb3 inflamed retinal pigment epithelial cells. We found lyso-Gb3 induced necroptosis of a retinal pigment epithelial cell line (ARPE-19) in an autophagy-dependent manner and that conditioned media (CM) from ARPE-19 cells treated with lyso-Gb3 induced the necroptosis, inflammation, and senescence of human umbilical vein endothelial cells. In addition, a pharmacological study showed CM from lyso-Gb3 treated ARPE-19 cells induced endothelial necroptosis, inflammation, and senescence were significantly inhibited by an autophagy inhibitor (3-MA) and by two necroptosis inhibitors (necrostatin and GSK-872), respectively. These results demonstrate lyso-Gb3 induces necroptosis via autophagy and suggest that lyso-Gb3 inflamed retinal pigment epithelial cells trigger endothelial dysfunction via the autophagy-dependent necroptosis pathway. This study suggests the involvement of a novel autophagy-dependent necroptosis pathway in the regulation of endothelial dysfunction in Fabry disease.
Figure
Reference
-
1. Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. 1967; Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 276:1163–1167. DOI: 10.1056/NEJM196705252762101. PMID: 6023233.2. Lücke T, Höppner W, Schmidt E, Illsinger S, Das AM. 2004; Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab. 82:93–97. DOI: 10.1016/j.ymgme.2004.01.011. PMID: 15110329.
Article3. Levine B, Klionsky DJ. 2004; Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 6:463–477. DOI: 10.1016/S1534-5807(04)00099-1. PMID: 15068787.4. Choi ME, Price DR, Ryter SW, Choi AMK. 2019; Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 4:e128834. DOI: 10.1172/jci.insight.128834. PMID: 31391333. PMCID: PMC6693822.
Article5. Pasparakis M, Vandenabeele P. 2015; Necroptosis and its role in inflammation. Nature. 517:311–320. DOI: 10.1038/nature14191. PMID: 25592536.
Article6. Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH. 2014; RIPK3 as a potential therapeutic target for Gaucher's disease. Nat Med. 20:204–208. DOI: 10.1038/nm.3449. PMID: 24441827.
Article7. Rodier F, Campisi J. 2011; Four faces of cellular senescence. J Cell Biol. 192:547–556. DOI: 10.1083/jcb.201009094. PMID: 21321098. PMCID: PMC3044123.
Article8. Nigro P, Abe J, Woo CH, Satoh K, McClain C, O'Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, Berk BC. 2010; PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 116:1971–1979. DOI: 10.1182/blood-2010-02-269134. PMID: 20538799. PMCID: PMC3173991.
Article9. Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. 2009; Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 4:1798–1806. DOI: 10.1038/nprot.2009.191. PMID: 20010931.
Article10. El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, Schoenlein PV, Cutler CW. 2015; Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 10:e1004647. DOI: 10.1371/journal.ppat.1004647. PMID: 25679217. PMCID: PMC4352937. PMID: 8c26c24211924711b92b30d02e63acb4.
Article11. Zhang JM, An J. 2007; Cytokines, inflammation, and pain. Int Anesthesiol Clin. 45:27–37. DOI: 10.1097/AIA.0b013e318034194e. PMID: 17426506. PMCID: PMC2785020.
Article12. Hanada T, Yoshimura A. 2002; Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 13:413–421. DOI: 10.1016/S1359-6101(02)00026-6. PMID: 12220554.
Article13. Chan AH, Schroder K. 2020; Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med. 217:e20190314. DOI: 10.1084/jem.20190314. PMID: 31611248. PMCID: PMC7037238.
Article14. van Eijk M, Ferraz MJ, Boot RG, Aerts JMFG. 2020; Lyso-glycosphingolipids: presence and consequences. Essays Biochem. 64:565–578. DOI: 10.1042/EBC20190090. PMID: 32808655. PMCID: PMC7517347.
Article15. Rozenfeld P, Feriozzi S. 2017; Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. 122:19–27. DOI: 10.1016/j.ymgme.2017.09.004. PMID: 28947349.
Article16. Sanchez-Niño MD, Carpio D, Sanz AB, Ruiz-Ortega M, Mezzano S, Ortiz A. 2015; Lyso-Gb3 activates Notch1 in human podocytes. Hum Mol Genet. 24:5720–5732. DOI: 10.1093/hmg/ddv291. PMID: 26206887.
Article17. Ferraz MJ, Kallemeijn WW, Mirzaian M, Herrera Moro D, Marques A, Wisse P, Boot RG, Willems LI, Overkleeft HS, Aerts JM. 2014; Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim Biophys Acta. 1841:811–825. DOI: 10.1016/j.bbalip.2013.11.004. PMID: 24239767.
Article18. Stepien KM, Roncaroli F, Turton N, Hendriksz CJ, Roberts M, Heaton RA, Hargreaves I. 2020; Mechanisms of mitochondrial dysfunction in lysosomal storage disorders: a review. J Clin Med. 9:2596. DOI: 10.3390/jcm9082596. PMID: 32796538. PMCID: PMC7463786. PMID: c0ad71572dab44ccb9a6096a74d2e4b0.
Article19. Sanchez-Niño MD, Sanz AB, Carrasco S, Saleem MA, Mathieson PW, Valdivielso JM, Ruiz-Ortega M, Egido J, Ortiz A. 2011; Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 26:1797–1802. DOI: 10.1093/ndt/gfq306. PMID: 20504837.
Article20. Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, Chen X, Liang Y, Wu J, Zhao S, Zhou D, Han J. 2018; RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 20:186–197. DOI: 10.1038/s41556-017-0022-y. PMID: 29358703.
Article21. Cougnoux A, Cluzeau C, Mitra S, Li R, Williams I, Burkert K, Xu X, Wassif CA, Zheng W, Porter FD. 2016; Necroptosis in Niemann-Pick disease, type C1: a potential therapeutic target. Cell Death Dis. 7:e2147. DOI: 10.1038/cddis.2016.16. PMID: 26986514. PMCID: PMC4823930.
Article22. Zhu K, Liang W, Ma Z, Xu D, Cao S, Lu X, Liu N, Shan B, Qian L, Yuan J. 2018; Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis. 9:500. DOI: 10.1038/s41419-018-0524-y. PMID: 29703889. PMCID: PMC5923285.
Article23. Zu R, Yu Z, Zhao J, Lu X, Liang W, Sun L, Si C, Zhu K, Zhang T, Li G, Zhang M, Zhang Y, Liu N, Yuan J, Shan B. 2021; Quantitative analysis of phosphoproteome in necroptosis reveals a role of TRIM28 phosphorylation in promoting necroptosis-induced cytokine production. Cell Death Dis. 12:994. DOI: 10.1038/s41419-021-04290-7. PMID: 34689152. PMCID: PMC8542044. PMID: 70ad286ccd1f4e56af15db5ed08e7b33.
Article24. Kaczmarek A, Vandenabeele P, Krysko DV. 2013; Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 38:209–223. DOI: 10.1016/j.immuni.2013.02.003. PMID: 23438821.
Article25. Jung S, Seo DJ, Yeo D, Wang Z, Min A, Zhao Z, Song M, Choi IS, Myoung J, Choi C. 2020; Experimental infection of hepatitis E virus induces pancreatic necroptosis in miniature pigs. Sci Rep. 10:12022. DOI: 10.1038/s41598-020-68959-3. PMID: 32694702. PMCID: PMC7374588.
Article26. Frank D, Vince JE. 2019; Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26:99–114. DOI: 10.1038/s41418-018-0212-6. PMID: 30341423. PMCID: PMC6294779.
Article27. Godo S, Shimokawa H. 2017; Endothelial functions. Arterioscler Thromb Vasc Biol. 37:e108–e114. DOI: 10.1161/ATVBAHA.117.309813. PMID: 28835487.
Article28. Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. 2019; Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 6:19. DOI: 10.3390/jcdd6020019. PMID: 31035613. PMCID: PMC6616540. PMID: 35945fd9b2c54bb092e36026be981167.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endothelial cell autophagy in the context of disease development
- The Role of Autophagy in Erectile Dysfunction
- Dual Roles of Autophagy and Their Potential Drugs for Improving Cancer Therapeutics
- Impairment of Mitochondrial ATP Synthesis Induces RIPK3-dependent Necroptosis in Lung Epithelial Cells During Lung Injury by Lung Inflammation
- Necroptosis in Liver and Pancreatic Diseases