Anat Cell Biol.
2023 Mar;56(1):16-24. 10.5115/acb.22.098.
Endothelial cell autophagy in the context of disease development
- Affiliations
-
- 1National Research Mordovia State University, Saransk, Russia
- KMID: 2540980
- DOI: http://doi.org/10.5115/acb.22.098
Abstract
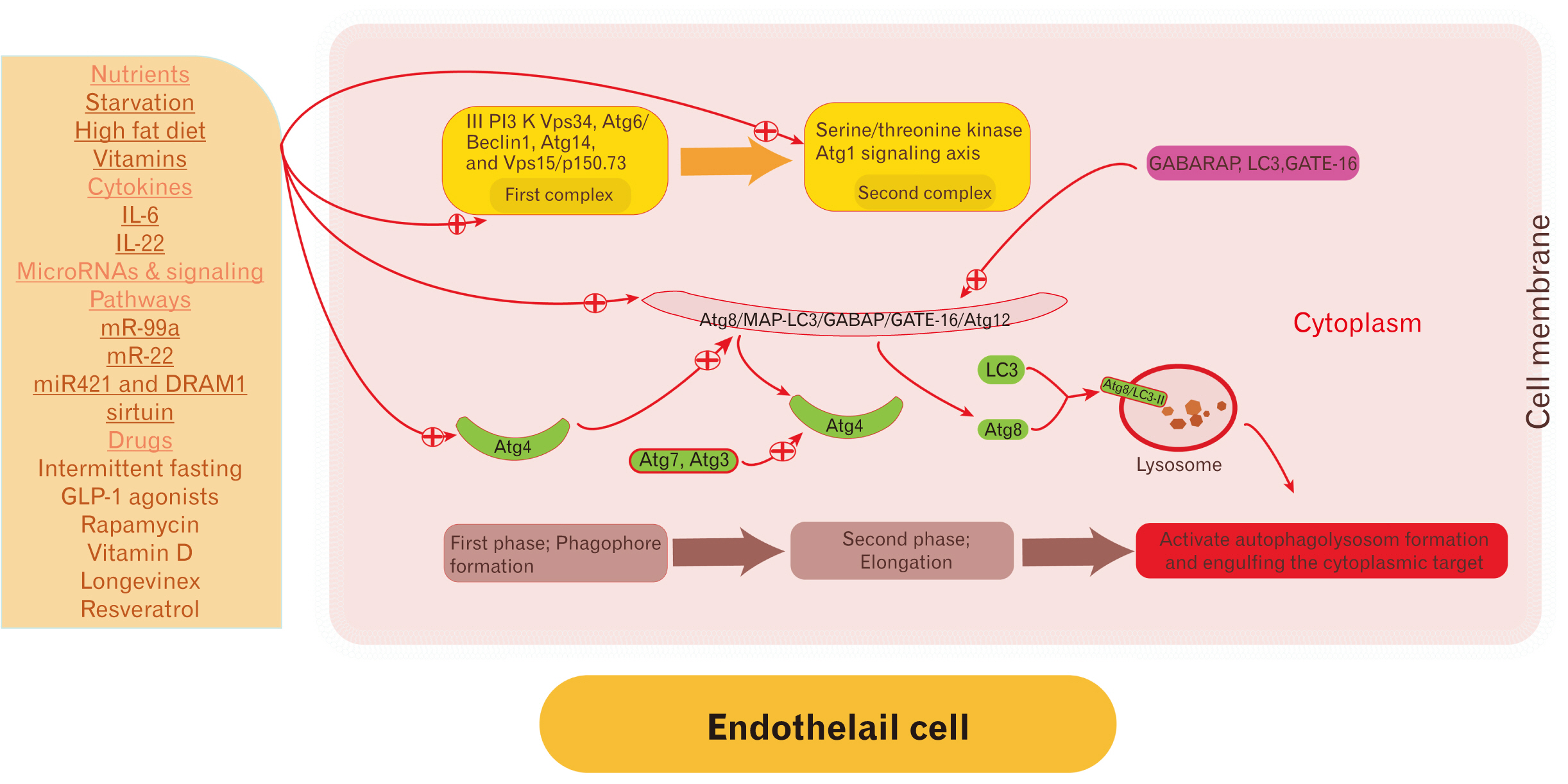
- Endothelial cells (EC) are the anatomical boundaries between the intravascular and extravascular space. Damage to ECs is catastrophic and induces endothelial cell dysfunction. The pathogenesis is multifactorial and involves dysregulation in the signaling pathways, membrane lipids ratio disturbance, cell-cell adhesion disturbance, unfolded protein response, lysosomal and mitochondrial stress, autophagy dysregulation, and oxidative stress. Autophagy is a lysosomal-dependent turnover of intracellular components. Autophagy was recognized early in the pathogenesis of endothelial dysfunction. Autophagy is a remarkable patho (physiological) process in the cell homeostasis regulation including EC. Regulation of autophagy rate is disease-dependent and impaired with aging. Up-regulation of autophagy induces endothelial cell regeneration/differentiation and improves the function of impaired ones. The paper scrutinizes the molecular mechanisms and triggers of EC dysregulation and current perspectives for future therapeutic strategies by autophagy targeting.
Keyword
Figure
Cited by 1 articles
-
Tree of life: endothelial cell in norm and disease, the good guy is a partner in crime!
Basheer Abdullah Marzoog
Anat Cell Biol. 2023;56(2):166-178. doi: 10.5115/acb.22.190.
Reference
-
References
1. Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P. 2017; Central role of metabolism in endothelial cell function and vascular disease. Physiology (Bethesda). 32:126–40. DOI: 10.1152/physiol.00031.2016. PMID: 28202623. PMCID: PMC5337830.
Article2. Khan S, Taverna F, Rohlenova K, Treps L, Geldhof V, de Rooij L, Sokol L, Pircher A, Conradi LC, Kalucka J, Schoonjans L, Eelen G, Dewerchin M, Karakach T, Li X, Goveia J, Carmeliet P. 2019; EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res. 47(D1):D736–44. DOI: 10.1093/nar/gky997. PMID: 30357379. PMCID: PMC6324065.
Article3. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. 2013; The vascular endothelium and human diseases. Int J Biol Sci. 9:1057–69. DOI: 10.7150/ijbs.7502. PMID: 24250251. PMCID: PMC3831119.
Article4. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. 2008; The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 15:261–71. DOI: 10.1016/j.devcel.2008.07.002. PMID: 18694565. PMCID: PMC2685763.
Article5. Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. 2020; Distinct types of cell death and the implication in diabetic cardiomyopathy. Front Pharmacol. 11:42. DOI: 10.3389/fphar.2020.00042. PMID: 32116717. PMCID: PMC7018666. PMID: bd22dd15e8ff4ec4beacaf4aa092b797.
Article6. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. 2018; Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25:486–541. DOI: 10.1038/s41418-017-0012-4. PMID: 29362479. PMCID: PMC5864239.
Article7. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. 2009; Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16:3–11. DOI: 10.1038/cdd.2008.150. PMID: 18846107. PMCID: PMC2744427.
Article8. Marzoog BA, Vlasova TI. 2022; Myocardiocyte autophagy in the context of myocardiocytes regeneration: a potential novel therapeutic strategy. Egypt J Med Hum Genet. 23:41. DOI: 10.1186/s43042-022-00250-8. PMID: d1da3ab8dd21477095572d54dc7e6bc3.
Article9. Joffre J, Hellman J, Ince C, Ait-Oufella H. 2020; Endothelial responses in sepsis. Am J Respir Crit Care Med. 202:361–70. DOI: 10.1164/rccm.201910-1911TR. PMID: 32101446.
Article10. Paone S, Baxter AA, Hulett MD, Poon IKH. 2019; Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci. 76:1093–106. DOI: 10.1007/s00018-018-2983-9. PMID: 30569278.
Article11. Morris G, Puri BK, Olive L, Carvalho A, Berk M, Walder K, Gustad LT, Maes M. 2020; Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 18:305. DOI: 10.1186/s12916-020-01749-w. PMID: 33070778. PMCID: PMC7570030. PMID: 2ab20273ea0c4e84a8a10b83ba3bcfa9.
Article12. Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. 2014; MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 20:368–76. DOI: 10.1038/nm.3487. PMID: 24584117. PMCID: PMC4398028.
Article13. Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N. 2013; Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 128:2026–38. DOI: 10.1161/CIRCULATIONAHA.113.001720. PMID: 24014835.
Article14. Masoud AG, Lin J, Azad AK, Farhan MA, Fischer C, Zhu LF, Zhang H, Sis B, Kassiri Z, Moore RB, Kim D, Anderson CC, Vederas JC, Adam BA, Oudit GY, Murray AG. 2020; Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J Clin Invest. 130:94–107. DOI: 10.1172/JCI128469. PMID: 31738185. PMCID: PMC6934203.
Article15. Xia W, Yin J, Zhang S, Guo C, Li Y, Zhang Y, Zhang A, Jia Z, Chen H. 2018; Parkin modulates ERRα/eNOS signaling pathway in endothelial cells. Cell Physiol Biochem. 49:2022–34. DOI: 10.1159/000493713. PMID: 30244249.
Article16. Kang S, Tanaka T, Inoue H, Ono C, Hashimoto S, Kioi Y, Matsumoto H, Matsuura H, Matsubara T, Shimizu K, Ogura H, Matsuura Y, Kishimoto T. 2020; IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A. 117:22351–6. DOI: 10.1073/pnas.2010229117. PMID: 32826331. PMCID: PMC7486751.
Article17. Cuervo AM, Wong E. 2014; Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 24:92–104. DOI: 10.1038/cr.2013.153. PMID: 24281265. PMCID: PMC3879702.
Article18. Suzuki K, Ohsumi Y. 2007; Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 581:2156–61. DOI: 10.1016/j.febslet.2007.01.096. PMID: 17382324.19. Dikic I, Elazar Z. 2018; Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 19:349–64. DOI: 10.1038/s41580-018-0003-4. PMID: 29618831.
Article20. Gatica D, Chiong M, Lavandero S, Klionsky DJ. 2015; Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 116:456–67. Erratum in: Circ Res 2015;116:e56. DOI: 10.1161/CIRCRESAHA.114.303788. PMID: 25634969. PMCID: PMC4313620.
Article21. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. 2010; Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 90:1383–435. DOI: 10.1152/physrev.00030.2009. PMID: 20959619.
Article22. Di Malta C, Cinque L, Settembre C. 2019; Transcriptional regulation of autophagy: mechanisms and diseases. Front Cell Dev Biol. 7:114. DOI: 10.3389/fcell.2019.00114. PMID: 31312633. PMCID: PMC6614182.
Article23. Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB 3rd. 2013; Autophagy: regulation and role in development. Autophagy. 9:951–72. DOI: 10.4161/auto.24273. PMID: 24121596. PMCID: PMC3722331.24. Icli A, Cure E, Cure MC, Uslu AU, Balta S, Mikhailidis DP, Ozturk C, Arslan S, Sakız D, Sahin M, Kucuk A. 2016; Endocan levels and subclinical atherosclerosis in patients with systemic lupus erythematosus. Angiology. 67:749–55. DOI: 10.1177/0003319715616240. PMID: 26614790.
Article25. Leite AR, Borges-Canha M, Cardoso R, Neves JS, Castro-Ferreira R, Leite-Moreira A. 2020; Novel biomarkers for evaluation of endothelial dysfunction. Angiology. 71:397–410. DOI: 10.1177/0003319720903586. PMID: 32077315.
Article26. Musialowska D, Zbroch E, Koc-Zorawska E, Musialowski P, Malyszko J. 2018; Endocan concentration in patients with primary hypertension. Angiology. 69:483–9. DOI: 10.1177/0003319717736158. PMID: 29084442.
Article27. Oral E, Halici Z, Cinar I, Ozcan E, Kutlu Z. 2019; Evaluation of endothelial dysfunction in bipolar affective disorders: serum endocan and urotensin-II levels. Clin Psychopharmacol Neurosci. 17:211–21. DOI: 10.9758/cpn.2019.17.2.211. PMID: 30905121. PMCID: PMC6478082.
Article28. Ibrahim SMA, Sabah HMA, Eldesoky AI, Soltan MY, Elshamy HA, Abdulhady H. 2022; Role of endothelial cell specific molecule-1 (endocan) and cardiac imaging in early detection of cardiac involvement in psoriatic patients with or without arthritis. Egypt Rheumatol. 44:159–64. DOI: 10.1016/j.ejr.2021.11.002.
Article29. Altintas N, Mutlu LC, Akkoyun DC, Aydin M, Bilir B, Yilmaz A, Malhotra A. 2016; Effect of CPAP on new endothelial dysfunction marker, endocan, in people with obstructive sleep apnea. Angiology. 67:364–74. DOI: 10.1177/0003319715590558. PMID: 26076702. PMCID: PMC4846580.
Article30. Balamir I, Ates I, Topcuoglu C, Turhan T. 2018; Association of endocan, ischemia-modified albumin, and hsCRP levels with endothelial dysfunction in type 2 diabetes mellitus. Angiology. 69:609–16. DOI: 10.1177/0003319717740781. PMID: 29172652.
Article31. Çimen T, Efe TH, Akyel A, Sunman H, Algül E, Şahan HF, Erden G, Özdemir Ş, Alay EF, Doğan M, Yeter E. 2016; Human endothelial cell-specific molecule-1 (Endocan) and coronary artery disease and microvascular angina. Angiology. 67:846–53. DOI: 10.1177/0003319715625827. PMID: 26744512.
Article32. Aparci M, Isilak Z, Uz O, Yalcin M, Kucuk U. 2015; Endocan and endothelial dysfunction. Angiology. 66:488–9. DOI: 10.1177/0003319714568791. PMID: 25632053.
Article33. Li X, Liu H, Zhang Y, Gu Y, Sun L, Yu H, Bai W. 2022; A prediction equation to estimate vascular endothelial function in different body mass index populations. Front Cardiovasc Med. 9:766565. DOI: 10.3389/fcvm.2022.766565. PMID: 35360015. PMCID: PMC8960173. PMID: 68c07f4677044d7b9b7da88a3981e63a.
Article34. Mudau M, Genis A, Lochner A, Strijdom H. 2012; Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 23:222–31. DOI: 10.5830/CVJA-2011-068. PMID: 22614668. PMCID: PMC3721957.35. Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. 2016; Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun. 7:12422. DOI: 10.1038/ncomms12422. PMID: 27516371. PMCID: PMC4990645. PMID: 442c956f54bf4c6eba0ef2653c390245.
Article36. Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. 2017; TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 18:2157. DOI: 10.3390/ijms18102157. PMID: 29039786. PMCID: PMC5666838.
Article37. Wong WT, Cooke JP. 2016; Therapeutic transdifferentiation of human fibroblasts into endothelial cells using forced expression of lineage-specific transcription factors. J Tissue Eng. 7:2041731416628329. DOI: 10.1177/2041731416628329. PMID: 27081470. PMCID: PMC4820020.
Article38. Marzoog BA, Vlasova TI. 2021; Beta-cell autophagy under the scope of hypoglycemic drugs; possible mechanism as a novel therapeutic target. Obe Metab. 18:465–70. DOI: 10.14341/omet12778.
Article39. Berendam SJ, Koeppel AF, Godfrey NR, Rouhani SJ, Woods AN, Rodriguez AB, Peske JD, Cummings KL, Turner SD, Engelhard VH. 2019; Comparative transcriptomic analysis identifies a range of immunologically related functional elaborations of lymph node associated lymphatic and blood endothelial cells. Front Immunol. 10:816. DOI: 10.3389/fimmu.2019.00816. PMID: 31057546. PMCID: PMC6478037.
Article40. Keuschnigg J, Karinen S, Auvinen K, Irjala H, Mpindi JP, Kallioniemi O, Hautaniemi S, Jalkanen S, Salmi M. 2013; Plasticity of blood- and lymphatic endothelial cells and marker identification. PLoS One. 8:e74293. DOI: 10.1371/journal.pone.0074293. PMID: 24058540. PMCID: PMC3769239. PMID: aa5f6f4cdead469a84ca43ce114f6dd0.
Article41. Yang J, Zhang S, Zhang L, Xie X, Wang H, Jie Z, Gu M, Yang JY, Cheng X, Sun SC. 2019; Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism. Cell Mol Immunol. 16:165–77. DOI: 10.1038/cmi.2017.167. PMID: 29503445. PMCID: PMC6355805.
Article42. Russo E, Runge P, Jahromi NH, Naboth H, Landtwing A, Montecchi R, Leicht N, Hunter MC, Takai Y, Halin C. 2021; CD112 regulates angiogenesis and T cell entry into the spleen. Cells. 10:169. DOI: 10.3390/cells10010169. PMID: 33467729. PMCID: PMC7830896. PMID: 48c9bb8f0a614e5d888c34399f17462b.
Article43. Claro V, Ferro A. 2020; Netrin-1: focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc Dis. 9:2048004020959574. DOI: 10.1177/2048004020959574. PMID: 33282228. PMCID: PMC7691900. PMID: 6d1ae565a2334a41a52233a84b211e6e.
Article44. Dieterich LC, Tacconi C, Menzi F, Proulx ST, Kapaklikaya K, Hamada M, Takahashi S, Detmar M. 2020; Lymphatic MAFB regulates vascular patterning during developmental and pathological lymphangiogenesis. Angiogenesis. 23:411–23. DOI: 10.1007/s10456-020-09721-1. PMID: 32307629. PMCID: PMC7311381.
Article45. Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, Rigothier C, Sallée M, Fremeaux-Bacchi V, Guerrot D, Roumenina LT. 2019; Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 15:87–108. DOI: 10.1038/s41581-018-0098-z. PMID: 30607032.
Article46. Becker PW, Sacilotto N, Nornes S, Neal A, Thomas MO, Liu K, Preece C, Ratnayaka I, Davies B, Bou-Gharios G, De Val S. 2016; An intronic Flk1 enhancer directs arterial-specific expression via RBPJ-mediated venous repression. Arterioscler Thromb Vasc Biol. 36:1209–19. DOI: 10.1161/ATVBAHA.116.307517. PMID: 27079877. PMCID: PMC4894770.47. Carman CV, Martinelli R. Bradshaw RA, Stahl PD, editors. 2016. Lymphocyte-endothelial interactions. Encyclopedia of Cell Biology. Elsevier;Waltham: p. 632–49. DOI: 10.1016/B978-0-12-394447-4.30095-5.
Article48. Ribatti D. Ribatti D, editor. 2017. The origins of lymphatic vessels: an historical review. Milestones in Immunology: Based on Collected Papers. Elsevier;London: p. 129–62. DOI: 10.1016/B978-0-12-811313-4.00010-3.49. Motawe ZY, Abdelmaboud SS, Breslin JW. 2021; Involvement of sigma receptor-1 in lymphatic endothelial barrier integrity and bioenergetic regulation. Lymphat Res Biol. 19:231–9. DOI: 10.1089/lrb.2020.0060. PMID: 33226886. PMCID: PMC8220569.
Article50. Park HS, Kim SY. 2021; Endothelial cell senescence: a machine learning-based meta-analysis of transcriptomic studies. Ageing Res Rev. 65:101213. DOI: 10.1016/j.arr.2020.101213. PMID: 33189866.
Article51. Li C, Tan Y, Wu J, Ma Q, Bai S, Xia Z, Wan X, Liang J. 2020; Resveratrol improves Bnip3-related mitophagy and attenuates high-fat-induced endothelial dysfunction. Front Cell Dev Biol. 8:796. DOI: 10.3389/fcell.2020.00796. PMID: 32923443. PMCID: PMC7457020. PMID: 373f21a8c3fe47b692cf70671f1ac543.
Article52. Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. 2006; Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 116:2333–43. DOI: 10.1172/JCI27154. PMID: 16955137. PMCID: PMC1555637.
Article53. Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. 2013; Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 33:1366–75. DOI: 10.1161/ATVBAHA.112.301167. PMID: 23520160. PMCID: PMC3898631.
Article54. Huang X, Zhang X, Zhao DX, Yin J, Hu G, Evans CE, Zhao YY. 2019; Endothelial hypoxia-inducible factor-1α is required for vascular repair and resolution of inflammatory lung injury through forkhead box protein M1. Am J Pathol. 189:1664–79. DOI: 10.1016/j.ajpath.2019.04.014. PMID: 31121134. PMCID: PMC6680254.
Article55. Liu M, Zhang L, Marsboom G, Jambusaria A, Xiong S, Toth PT, Benevolenskaya EV, Rehman J, Malik AB. 2019; Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat Commun. 10:2126. DOI: 10.1038/s41467-019-10134-y. PMID: 31073164. PMCID: PMC6509327. PMID: aef5d536288346a9aed9848b8a1f6268.
Article56. McDonald AI, Shirali AS, Aragón R, Ma F, Hernandez G, Vaughn DA, Mack JJ, Lim TY, Sunshine H, Zhao P, Kalinichenko V, Hai T, Pelegrini M, Ardehali R, Iruela-Arispe ML. 2018; Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell. 23:210–25.e6. DOI: 10.1016/j.stem.2018.07.011. PMID: 30075129. PMCID: PMC6178982.
Article57. Huang X, Dai Z, Cai L, Sun K, Cho J, Albertine KH, Malik AB, Schraufnagel DE, Zhao YY. 2016; Endothelial p110γPI3K mediates endothelial regeneration and vascular repair after inflammatory vascular injury. Circulation. 133:1093–103. DOI: 10.1161/CIRCULATIONAHA.115.020918. PMID: 26839042. PMCID: PMC4792690.
Article58. Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A, Rashid ST, Futers TS, Xuan S, Gatenby VK, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. 2012; Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes. 61:2359–68. DOI: 10.2337/db11-1494. PMID: 22733797. PMCID: PMC3425420.59. Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, Fuster V, Badimon JJ, Sauter BV. 2004; Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 110:2430–5. DOI: 10.1161/01.CIR.0000145120.37891.8A. PMID: 15477421.
Article60. Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. 2008; Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 103:432–40. DOI: 10.1161/CIRCRESAHA.108.179333. PMID: 18617693.
Article61. Miyagawa K, Shi M, Chen PI, Hennigs JK, Zhao Z, Wang M, Li CG, Saito T, Taylor S, Sa S, Cao A, Wang L, Snyder MP, Rabinovitch M. 2019; Smooth muscle contact drives endothelial regeneration by BMPR2-Notch1-mediated metabolic and epigenetic changes. Circ Res. 124:211–24. DOI: 10.1161/CIRCRESAHA.118.313374. PMID: 30582451. PMCID: PMC6400637.
Article62. Tang X, Luo YX, Chen HZ, Liu DP. 2014; Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 5:175. DOI: 10.3389/fphys.2014.00175. PMID: 24834056. PMCID: PMC4018556.
Article63. Kumar V, Jurkunas UV. 2021; Mitochondrial dysfunction and mitophagy in Fuchs endothelial corneal dystrophy. Cells. 10:1888. DOI: 10.3390/cells10081888. PMID: 34440658. PMCID: PMC8392447. PMID: f86c6b57bb624613bd9804c11cdc89ba.
Article64. Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, Kremastinos D, Landmesser U, Protogerou A, Stefanadis C, Tousoulis D, Vassalli G, Vink H, Werner N, Wilkinson I, Vlachopoulos C. 2011; Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 18:775–89. DOI: 10.1177/1741826711398179. PMID: 21450600.
Article65. Shao X, Lai D, Zhang L, Xu H. 2016; Induction of autophagy and apoptosis via PI3K/AKT/TOR pathways by azadirachtin A in Spodoptera litura cells. Sci Rep. 6:35482. DOI: 10.1038/srep35482. PMID: 27752103. PMCID: PMC5067515.
Article66. Chen Y, Li P, Peng Y, Xie X, Zhang Y, Jiang Y, Li T, Qin X, Li S, Yang H, Wu C, Zheng C, Zhu J, You F, Liu Y. 2021; Protective autophagy attenuates soft substrate-induced apoptosis through ROS/JNK signaling pathway in breast cancer cells. Free Radic Biol Med. 172:590–603. DOI: 10.1016/j.freeradbiomed.2021.07.005. PMID: 34242793.
Article67. He C, Zhu H, Li H, Zou MH, Xie Z. 2013; Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 62:1270–81. DOI: 10.2337/db12-0533. PMID: 23223177. PMCID: PMC3609561.
Article68. Martin-Sanchez D, Poveda J, Fontecha-Barriuso M, Ruiz-Andres O, Sanchez-Niño MD, Ruiz-Ortega M, Ortiz A, Sanz AB. 2018; Targeting of regulated necrosis in kidney disease. Nefrologia (Engl Ed). 38:125–35. DOI: 10.1016/j.nefroe.2018.02.004. PMID: 28647049.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Autophagy in the Pathogenesis of Atherosclerosis
- The role of autophagy in cell proliferation and differentiation during tooth development
- Role of Autophagy in the Control of Cell Death and Inflammation
- Lyso-globotriaosylsphingosine induces endothelial dysfunction via autophagy-dependent regulation of necroptosis
- The role of autophagy in the placenta as a regulator of cell death