Korean J Physiol Pharmacol.
2021 Jan;25(1):87-94. 10.4196/kjpp.2021.25.1.87.
Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes
- Affiliations
-
- 1Department of Physiology, Dongguk University College of Medicine, Gyeongju 38066, Korea
- 2Channelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 10326, Korea
- 3Avixgen, Seoul 06649, Korea
- 4Department of Internal Medicine, Graduate School of Medicine, Dongguk University, Goyang 10326, Korea
- KMID: 2509658
- DOI: http://doi.org/10.4196/kjpp.2021.25.1.87
Abstract
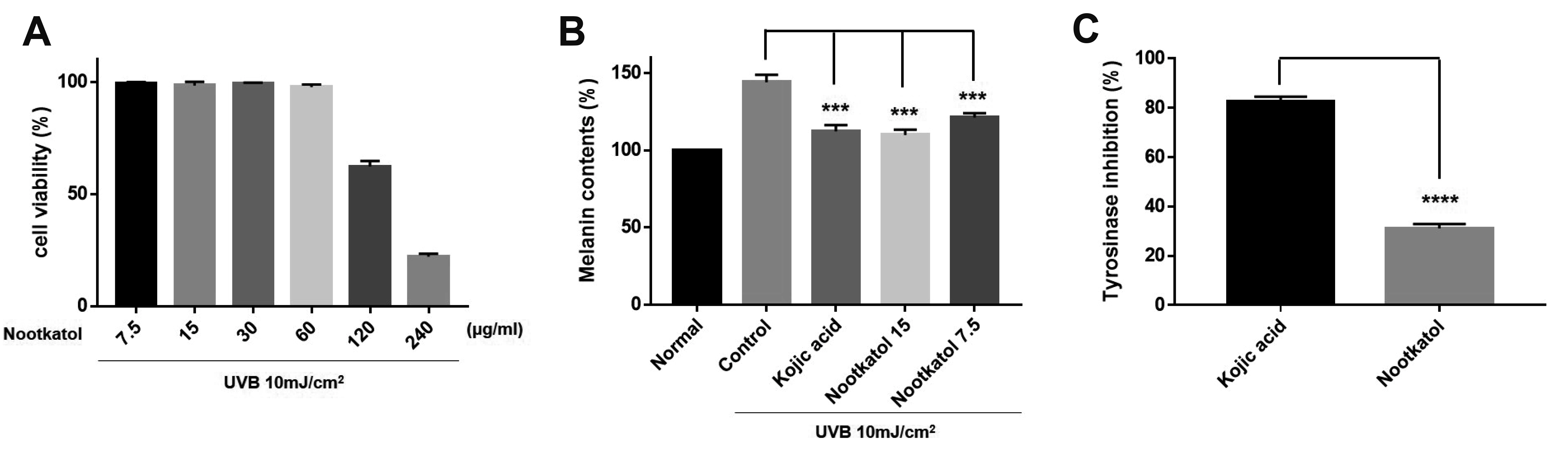
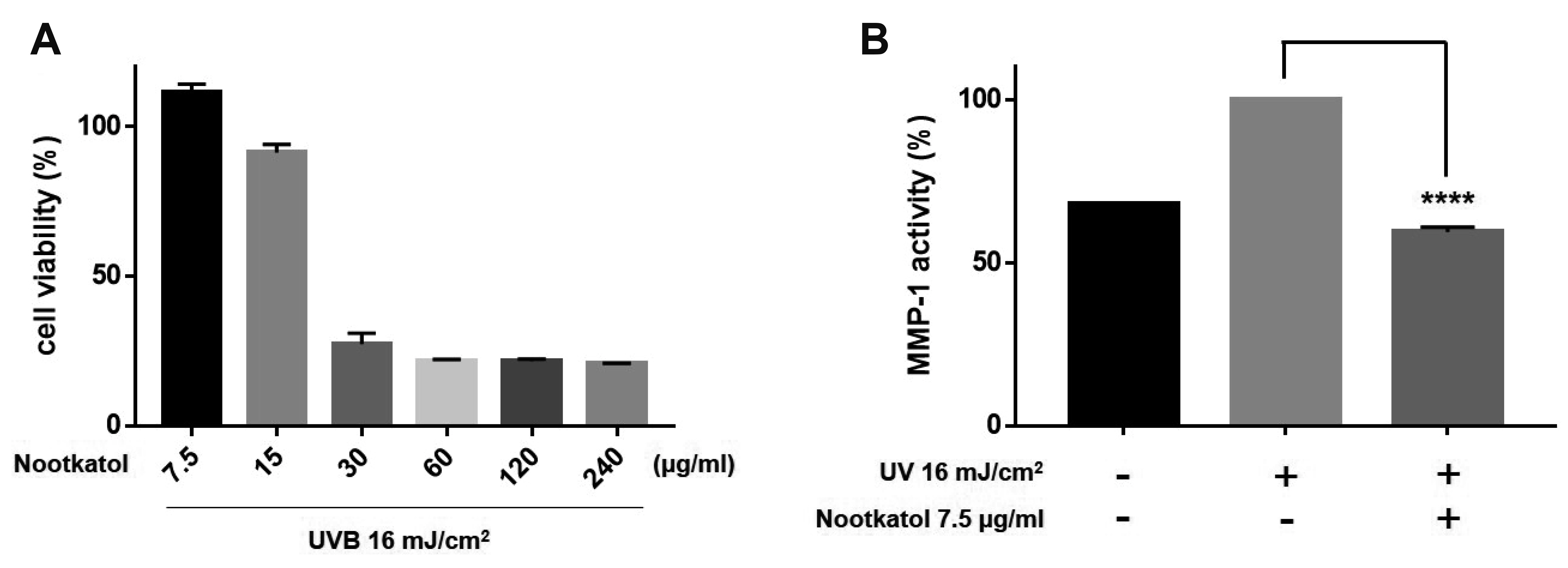
- Skin photoaging occurs due to chronic exposure to solar ultraviolet radiation (UV), the main factor contributing to extrinsic skin aging. Clinical signs of photoaging include the formation of deep, coarse skin wrinkles and hyperpigmentation. Although melanogenesis and skin wrinkling occur in different skin cells and have different underlying mechanisms, their initiation involves intracellular calcium signaling via calcium ion channels. The ORAI1 channel initiates melanogenesis in melanocytes, and the TRPV1 channel initiates MMP-1 production in keratinocytes in response to UV stimulation. We aimed to develop a drug that may simultaneously inhibit ORAI1 and TRPV1 activity to help prevent photoaging. We synthesized nootkatol, a chemical derivative of valencene. TRPV1 and ORAI1 activities were measured using the whole-cell patch-clamp technique. Intracellular calcium concentration [Ca2+ ] i was measured using calcium-sensitive fluorescent dye (Fura-2 AM). UV-induced melanin formation and MMP-1 production were quantified in B16F10 melanoma cells and HaCaT cells, respectively. Our results indicate that nootkatol (90 μM) reduced TRPV1 current by 94% ± 2% at –60 mV and ORAI1 current by 97% ± 1% at –120 mV. Intracellular calcium signaling was significantly inhibited by nootkatol in response to ORAI1 activation in human primary melanocytes (51.6% ± 0.98% at 100 μM). Additionally, UV-induced melanin synthesis was reduced by 76.38% ± 5.90% in B16F10 melanoma cells, and UV-induced MMP-1 production was reduced by 59.33% ± 1.49% in HaCaT cells. In conclusion, nootkatol inhibits both TRPV1 and ORAI1 to prevent photoaging, and targeting ion channels may be a promising strategy for preventing photoaging.
Keyword
Figure
Cited by 1 articles
-
Flos magnoliae constituent fargesin has an anti-allergic effectvia ORAI1 channel inhibition
Phan Thi Lam Hong, Hyun Jong Kim, Woo Kyung Kim, Joo Hyun Nam
Korean J Physiol Pharmacol. 2021;25(3):251-258. doi: 10.4196/kjpp.2021.25.3.251.
Reference
-
1. Gupta MA, Gilchrest BA. 2005; Psychosocial aspects of aging skin. Dermatol Clin. 23:643–648. DOI: 10.1016/j.det.2005.05.012. PMID: 16112440.
Article2. Shanbhag S, Nayak A, Narayan R, Nayak UY. 2019; Anti-aging and sunscreens: paradigm shift in cosmetics. Adv Pharm Bull. 9:348–359. DOI: 10.15171/apb.2019.042. PMID: 31592127. PMCID: PMC6773941.
Article3. Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J. 2015; Evaluation of skin ageing: a systematic review of clinical scales. Br J Dermatol. 172:1249–1261. DOI: 10.1111/bjd.13509. PMID: 25363020.
Article4. Farage MA, Miller KW, Elsner P, Maibach HI. 2008; Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 30:87–95. DOI: 10.1111/j.1468-2494.2007.00415.x. PMID: 18377617.
Article5. Kohl E, Steinbauer J, Landthaler M, Szeimies RM. 2011; Skin ageing. J Eur Acad Dermatol Venereol. 25:873–884. DOI: 10.1111/j.1468-3083.2010.03963.x. PMID: 21261751.
Article6. Amaro-Ortiz A, Yan B, D'Orazio JA. 2014; Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules. 19:6202–6219. DOI: 10.3390/molecules19056202. PMID: 24838074. PMCID: PMC4344124.
Article7. Rattanawiwatpong P, Wanitphakdeedecha R, Bumrungpert A, Maiprasert M. 2020; Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: a split-face, randomized controlled trial. J Cosmet Dermatol. 19:671–676. DOI: 10.1111/jocd.13305. PMID: 31975502. PMCID: PMC7027822.
Article8. Gilchrest BA. 2013; Photoaging. J Invest Dermatol. 133(E1):E2–E6. DOI: 10.1038/skinbio.2013.176. PMID: 23820721.
Article9. Krutmann J, Morita A, Chung JH. 2012; Sun exposure: what molecular photodermatology tells us about its good and bad sides. J Invest Dermatol. 132(3 Pt 2):976–984. DOI: 10.1038/jid.2011.394. PMID: 22170486.
Article10. Park HY, Kosmadaki M, Yaar M, Gilchrest BA. 2009; Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 66:1493–1506. DOI: 10.1007/s00018-009-8703-8. PMID: 19153661.
Article11. Schieke SM. 2003; [Photoaging and infrared radiation. Novel aspects of molecular mechanisms]. Hautarzt. 54:822–824. German. DOI: 10.1007/s00105-003-0577-3. PMID: 12955258.12. Choi TY, Park SY, Jo JY, Kang G, Park JB, Kim JG, Hong SG, Kim CD, Lee JH, Yoon TJ. 2009; Endogenous expression of TRPV1 channel in cultured human melanocytes. J Dermatol Sci. 56:128–130. DOI: 10.1016/j.jdermsci.2009.07.002. PMID: 19656659.
Article13. Kusumaningrum N, Lee DH, Yoon HS, Kim YK, Park CH, Chung JH. 2018; Gasdermin C is induced by ultraviolet light and contributes to MMP-1 expression via activation of ERK and JNK pathways. J Dermatol Sci. 90:180–189. DOI: 10.1016/j.jdermsci.2018.01.015. PMID: 29428815.
Article14. Kusumaningrum N, Lee DH, Yoon HS, Park CH, Chung JH. 2018; Ultraviolet light-induced gasdermin C expression is mediated via TRPV1/calcium/calcineurin/NFATc1 signaling. Int J Mol Med. 42:2859–2866. DOI: 10.3892/ijmm.2018.3839. PMID: 30226565.
Article15. Lee DU, Weon KY, Nam DY, Nam JH, Kim WK. 2016; Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp Dermatol. 25:977–982. DOI: 10.1111/exd.13151. PMID: 27488812.
Article16. Nam JH, Lee DU. 2015; Inhibitory effect of oleanolic acid from the rhizomes of Cyperus rotundus on transient receptor potential vanilloid 1 channel. Planta Med. 81:20–25. DOI: 10.1055/s-0034-1383304. PMID: 25402944.
Article17. Nam JH, Lee DU. 2016; Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 84:305–313. DOI: 10.1016/j.jdermsci.2016.09.017. PMID: 27712859.
Article18. Nam JH, Nam DY, Lee DU. 2016; Valencene from the rhizomes of Cyperus rotundus inhibits skin photoaging-related ion channels and Uv-induced melanogenesis in B16F10 melanoma cells. J Nat Prod. 79:1091–1096. DOI: 10.1021/acs.jnatprod.5b01127. PMID: 26967731.
Article19. Motiani RK, Tanwar J, Raja DA, Vashisht A, Khanna S, Sharma S, ivastava S Sr, Sivasubbu S, Natarajan VT, Gokhale RS. 2018; STIM1 activation of adenylyl cyclase 6 connects Ca2+ and cAMP signaling during melanogenesis. EMBO J. 37:e97597. DOI: 10.15252/embj.201797597. PMID: 29311116. PMCID: PMC5830913.
Article20. Stanisz H, Stark A, Kilch T, Schwarz EC, Müller CS, Peinelt C, Hoth M, Niemeyer BA, Vogt T, Bogeski I. 2012; ORAI1 Ca2+ channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol. 132:1443–1451. DOI: 10.1038/jid.2011.478. PMID: 22318387.21. Stanisz H, Vultur A, Herlyn M, Roesch A, Bogeski I. 2016; The role of Orai-STIM calcium channels in melanocytes and melanoma. J Physiol. 594:2825–2835. DOI: 10.1113/JP271141. PMID: 26864956. PMCID: PMC4887671.
Article22. Lee YM, Kim YK, Chung JH. 2009; Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp Dermatol. 18:431–436. DOI: 10.1111/j.1600-0625.2008.00806.x. PMID: 19161409.23. Lee YM, Kang SM, Chung JH. 2012; The role of TRPV1 channel in aged human skin. J Dermatol Sci. 65:81–85. DOI: 10.1016/j.jdermsci.2011.11.003. PMID: 22154816.
Article24. Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF, Zhou XJ. 2007; Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol. 16:985–992. DOI: 10.1111/j.1600-0625.2007.00619.x. PMID: 18031457.
Article25. Lee SE, Lee SH. 2018; Skin barrier and calcium. Ann Dermatol. 30:265–275. DOI: 10.5021/ad.2018.30.3.265. PMID: 29853739. PMCID: PMC5929942.
Article26. Tajima R, Oozeki H, Muraoka S, Tanaka S, Motegi Y, Nihei H, Yamada Y, Masuoka N, Nihei K. 2011; Synthesis and evaluation of bibenzyl glycosides as potent tyrosinase inhibitors. Eur J Med Chem. 46:1374–1381. DOI: 10.1016/j.ejmech.2011.01.065. PMID: 21334791.
Article27. Bellono NW, Oancea EV. 2014; Ion transport in pigmentation. Arch Biochem Biophys. 563:35–41. DOI: 10.1016/j.abb.2014.06.020. PMID: 25034214. PMCID: PMC4497562.
Article28. Caterina MJ, Pang Z. 2016; TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 9:77. DOI: 10.3390/ph9040077. PMID: 27983625. PMCID: PMC5198052.
Article29. Elsholz F, Harteneck C, Muller W, Friedland K. 2014; Calcium--a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol. 24:650–661. DOI: 10.1684/ejd.2014.2452. PMID: 25514792.30. Abdel-Malek ZA, Kadekaro AL, Swope VB. 2010; Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 23:171–186. DOI: 10.1111/j.1755-148X.2010.00679.x. PMID: 20128873.
Article31. Lee AY. 2015; Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 28:648–660. DOI: 10.1111/pcmr.12404. PMID: 26230865.
Article32. Schallreuter KU, Kothari S, Chavan B, Spencer JD. 2008; Regulation of melanogenesis--controversies and new concepts. Exp Dermatol. 17:395–404. DOI: 10.1111/j.1600-0625.2007.00675.x. PMID: 18177348.33. Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. 2011; UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 21:1906–1911. DOI: 10.1016/j.cub.2011.09.047. PMID: 22055294. PMCID: PMC3586554.
Article34. Bellono NW, Kammel LG, Zimmerman AL, Oancea E. 2013; UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A. 110:2383–2388. DOI: 10.1073/pnas.1215555110. PMID: 23345429. PMCID: PMC3568351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Supernatant from UVB - Irradiated Cultured Keratinocytes on the Growth , Melanin Content , and Tyrosinase Activity of Human Melanocyte
- Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha
- Side Effects of Suntan
- The Effects of Cytoskeletons on the Cultured Human Melanocytes
- p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts