Clin Exp Otorhinolaryngol.
2019 May;12(2):181-189. 10.21053/ceo.2018.00493.
Endoplasmic Reticulum Stress Induces MUC5AC and MUC5B Expression in Human Nasal Airway Epithelial Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck surgery, College of Medicine, Yeungnam University, Daegu, Korea. ydkim@med.yu.ac.kr
- 2Regional Center for Respiratory Diseases, Yeungnam University Medical Center, Daegu, Korea.
- KMID: 2447427
- DOI: http://doi.org/10.21053/ceo.2018.00493
Abstract
OBJECTIVES
Endoplasmic reticulum (ER) stress is known to be associated with inflammatory airway diseases, and three major transmembrane receptors: double-stranded RNA-activated protein kinase-like ER kinase, inositol requiring enzyme 1, and activating transcription factor 6 (ATF6) play important roles in ER stress-related proinflammatory signaling. However, the effects of ER stress and these three major signaling pathways on the regulation of the production of airway mucins in human nasal airway epithelial cells have not been elucidated.
METHODS
In primary human nasal epithelial cells, the effect of tunicamycin (an ER stress inducer) and 4-phenylbutyric acid (4-PBA, ER stress inhibitor) on the expression of MUC5AC and MUC5B was investigated by reverse transcriptasepolymerase chain reaction, real-time polymerase chain reaction, enzyme immunoassay, and immunoblot analysis. Small interfering RNA (siRNA) transfection was used to identify the mechanisms involved.
RESULTS
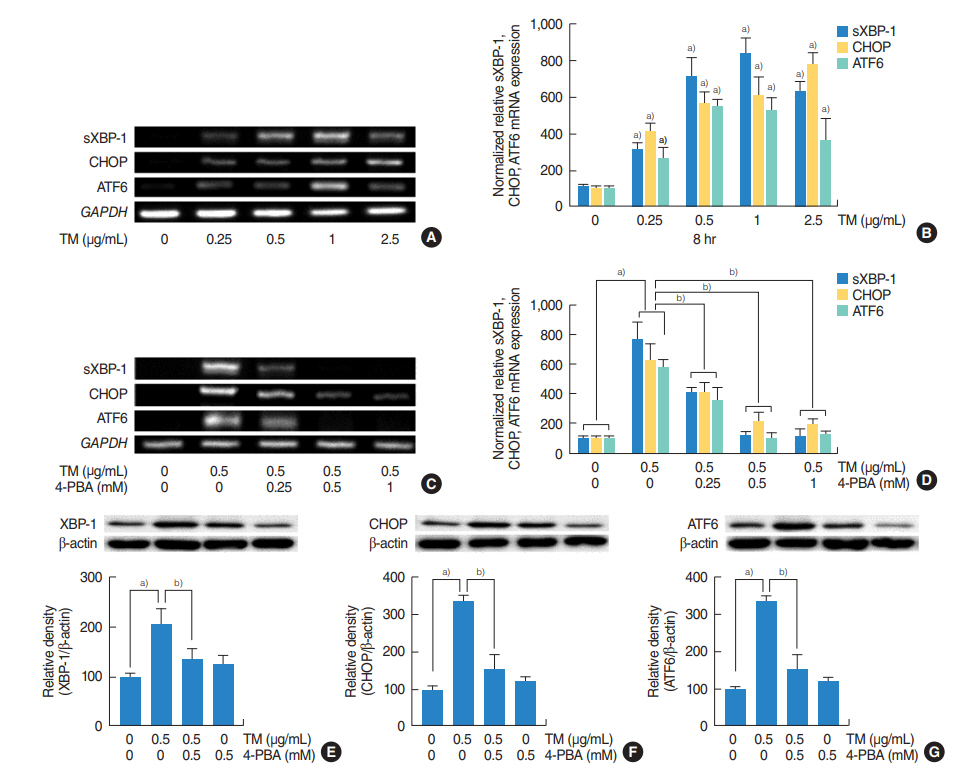
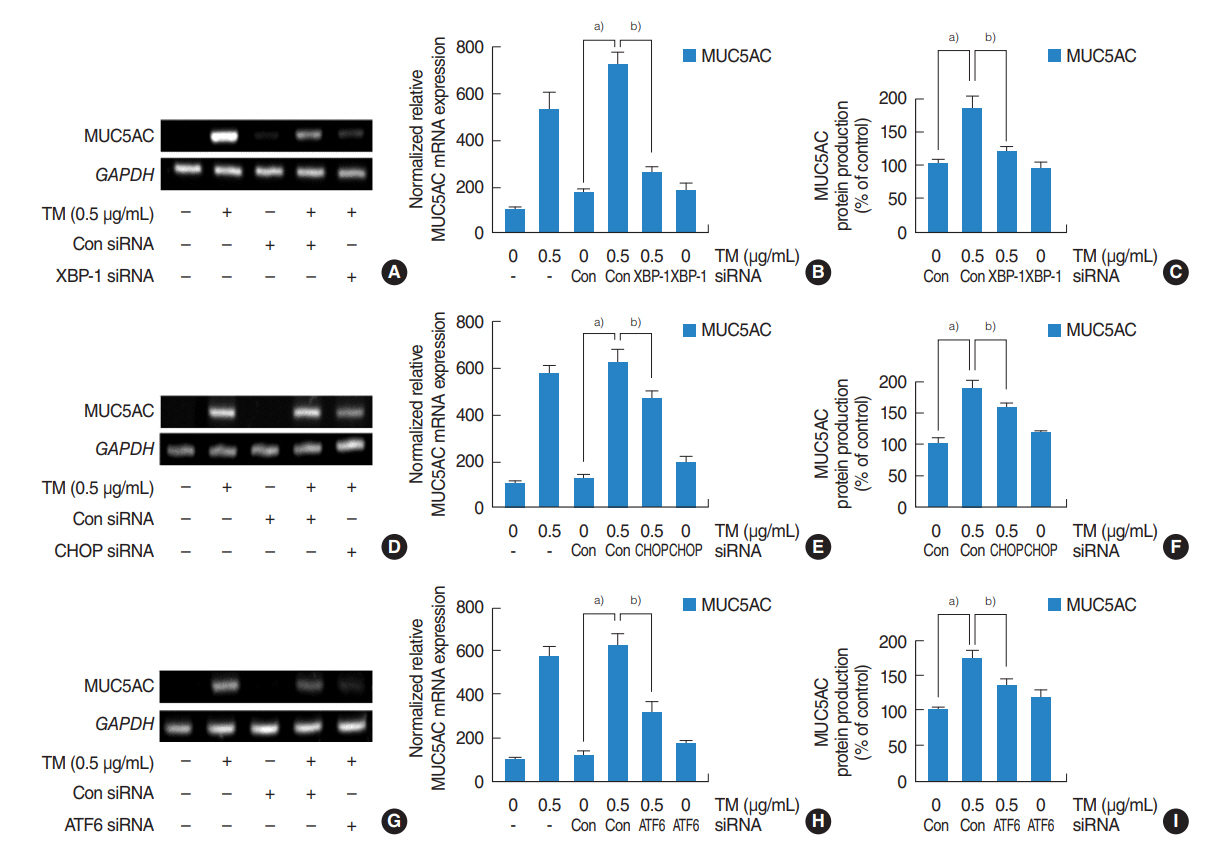
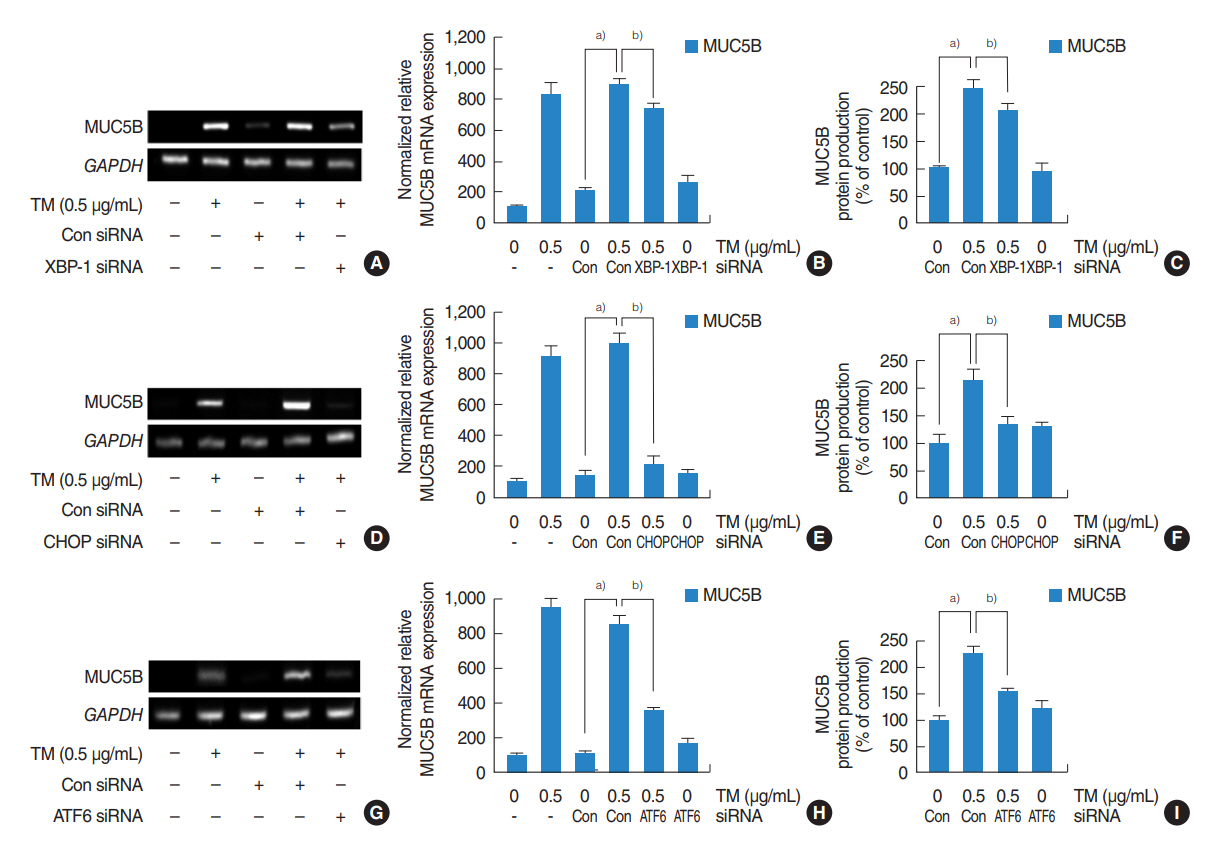
Tunicamycin increased the expressions of MUC5AC and MUC5B and the mRNA expressions of ER stress-related signaling molecules, including spliced X-box binding protein 1 (XBP-1), transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP), and ATF6. In addition, 4-PBA attenuated the tunicamycin-induced expressions of MUC5AC and MUC5B and the mRNA expressions of ER stress-related signaling molecules. Furthermore, siRNA knockdowns of XBP-1, CHOP, and ATF6 blocked the tunicamycin-induced mRNA expressions and glycoprotein productions of MUC5AC and MUC5B.
CONCLUSION
.: These results demonstrate that ER stress plays an important role in the regulation of MUC5AC and MUC5B via the activations of XBP-1, CHOP, and ATF6 in human nasal airway epithelial cells.
Keyword
MeSH Terms
-
Activating Transcription Factor 6
Carrier Proteins
CCAAT-Enhancer-Binding Proteins
Endoplasmic Reticulum Stress*
Endoplasmic Reticulum*
Epithelial Cells*
Glycoproteins
Humans*
Immunoenzyme Techniques
Inositol
Mucins
Phosphotransferases
Real-Time Polymerase Chain Reaction
RNA, Messenger
RNA, Small Interfering
Transcription Factor CHOP
Transcription Factors
Transfection
Tunicamycin
Activating Transcription Factor 6
CCAAT-Enhancer-Binding Proteins
Carrier Proteins
Glycoproteins
Inositol
Mucins
Phosphotransferases
RNA, Messenger
RNA, Small Interfering
Transcription Factor CHOP
Transcription Factors
Tunicamycin
Figure
Reference
-
1. Cao SS, Luo KL, Shi L. Endoplasmic reticulum stress interacts with inflammation in human diseases. J Cell Physiol. 2016; Feb. 231(2):288–94.
Article2. Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012; Mar. 90(3):260–70.
Article3. Kim SR, Lee YC. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy Asthma Immunol Res. 2015; Mar. 7(2):106–17.
Article4. Wei J, Rahman S, Ayaub EA, Dickhout JG, Ask K. Protein misfolding and endoplasmic reticulum stress in chronic lung disease. Chest. 2013; Apr. 143(4):1098–105.
Article5. Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, et al. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013; May. 6(3):639–54.
Article6. Mijosek V, Lasitschka F, Warth A, Zabeck H, Dalpke AH, Weitnauer M. Endoplasmic reticulum stress is a danger signal promoting innate inflammatory responses in bronchial epithelial cells. J Innate Immun. 2016; Aug. 8(5):464–78.
Article7. Delmotte P, Sieck GC. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca(2+) regulation in airway smooth muscle (ASM). Can J Physiol Pharmacol. 2015; Feb. 93(2):97–110.
Article8. Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007; May. 117(5):932–8.
Article9. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006; Jan. 86(1):245–78.
Article10. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014; Jan. 505(7483):412–6.
Article11. Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology. 2016; Jan. 97(1-2):84–100.
Article12. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009; Feb. 135(2):505–12.
Article13. Song SY, Woo HJ, Bae CH, Kim YW, Kim YD. Expression of leptin receptor in nasal polyps: leptin as a mucosecretagogue. Laryngoscope. 2010; May. 120(5):1046–50.
Article14. Kim YD, Bae CH, Song SY, Choi YS. Effect of beta-glucan on MUC4 and MUC5B expression in human airway epithelial cells. Int Forum Allergy Rhinol. 2015; Aug. 5(8):708–15.15. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; Jul. 8(7):519–29.
Article16. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004; Apr. 11(4):381–9.
Article17. Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression. Trends Mol Med. 2012; Oct. 18(10):589–98.
Article18. Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces ndoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. 2008; Aug. 8:229.
Article19. Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, et al. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J Allergy Clin Immunol. 2013; Dec. 132(6):1397–408.20. Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O’Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem. 2009; May. 284(22):14904–13.
Article21. Kim YM, Jin J, Choi JA, Cho SN, Lim YJ, Lee JH, et al. Staphylococcus aureus enterotoxin B-induced endoplasmic reticulum stress response is associated with chronic rhinosinusitis with nasal polyposis. Clin Biochem. 2014; Jan. 47(1-2):96–103.
Article22. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, et al. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012; Aug. 47(2):178–85.
Article23. Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002; Feb. 361(Pt 3):537–46.
Article24. Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006; Jan. 12(1):1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Multi-Walled Carbon Nanotubes on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Roflumilast Attenuates MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Peroxiredoxin 2 Inhibits Lipopolysaccharide Induced Mucin Expression and Reactive Oxygen Species Production in Human Airway Epithelial Cells
- Effect of High-Insulin on MUC4, MUC5AC, and MUC5B Expression in Airway Epithelial Cells
- Roxithromycin Suppresses MUC5B/8 Mucin Genes and Mucin Production in Airway Epithelial Cells