Nat Prod Sci.
2016 Dec;22(4):246-251. 10.20307/nps.2016.22.4.246.
Effects of (−)-Sesamin on Memory Deficits in MPTP-lesioned Mouse Model of Parkinson's Disease
- Affiliations
-
- 1Department of Pharmacy and Research Center for Bioresource and Health, College of Pharmacy, Chungbuk National University, 1, Chungdae-ro, Seowon-gu, Cheongju 28644, Korea. myklee@chungbuk.ac.kr
- KMID: 2366691
- DOI: http://doi.org/10.20307/nps.2016.22.4.246
Abstract
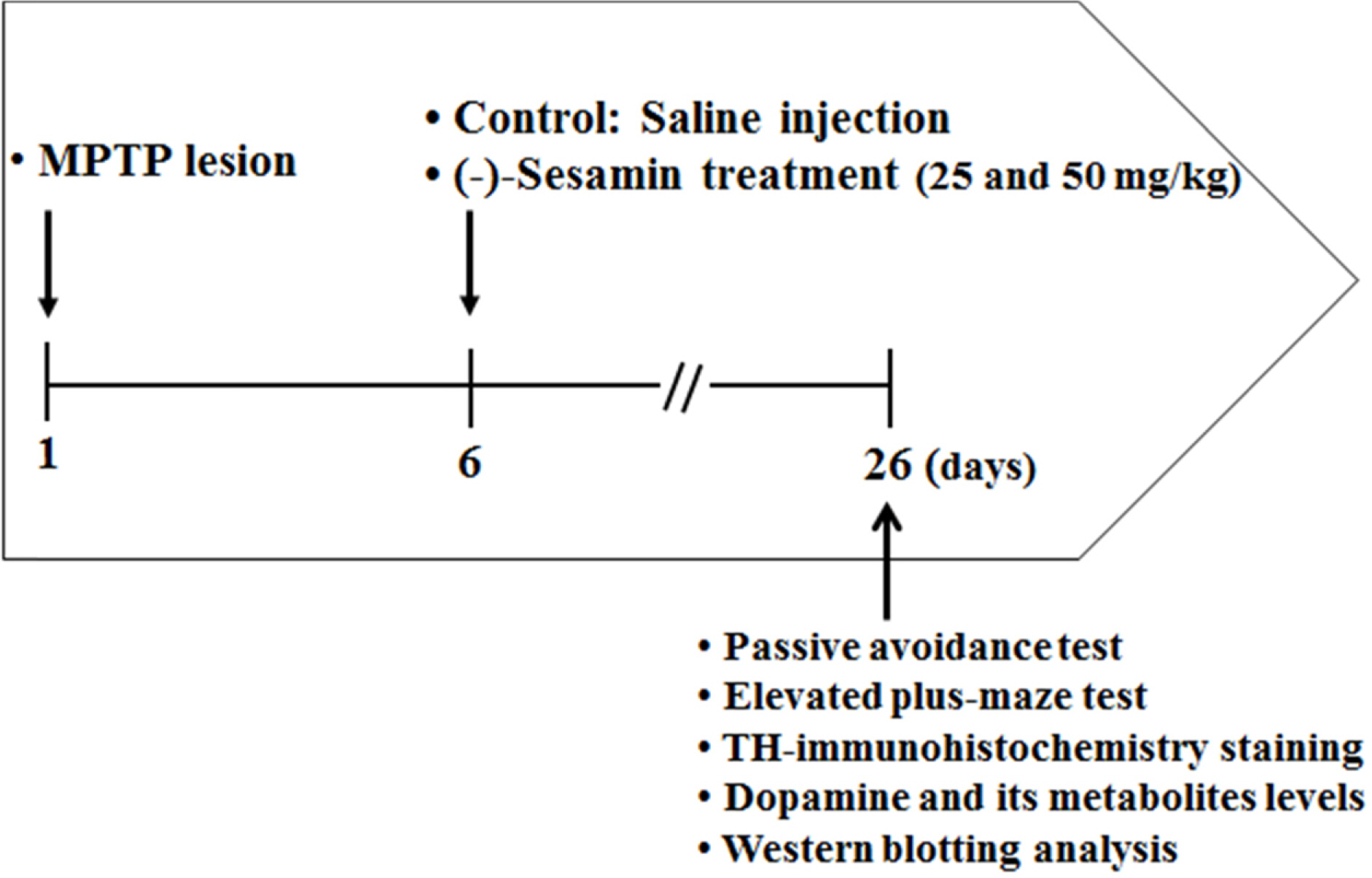
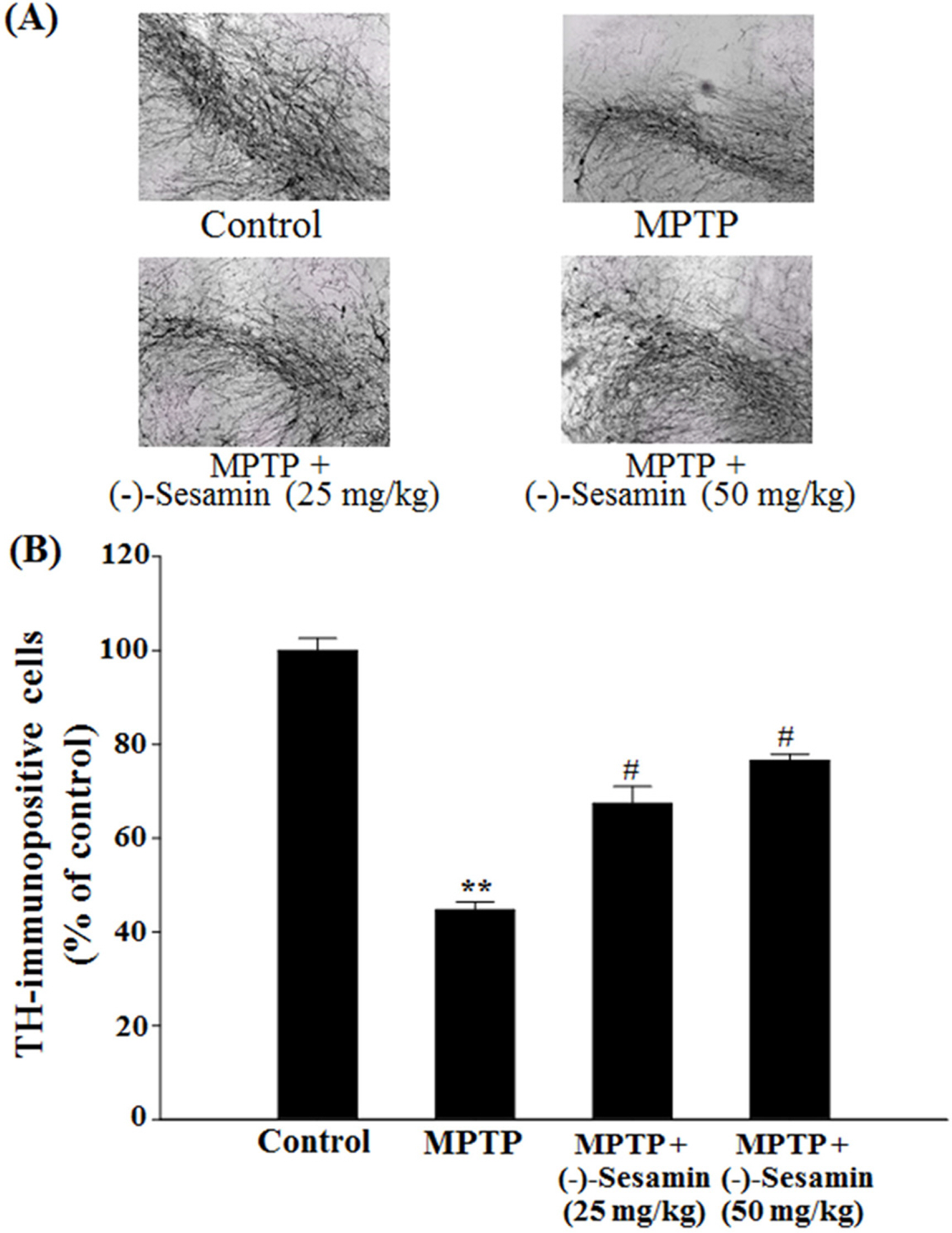
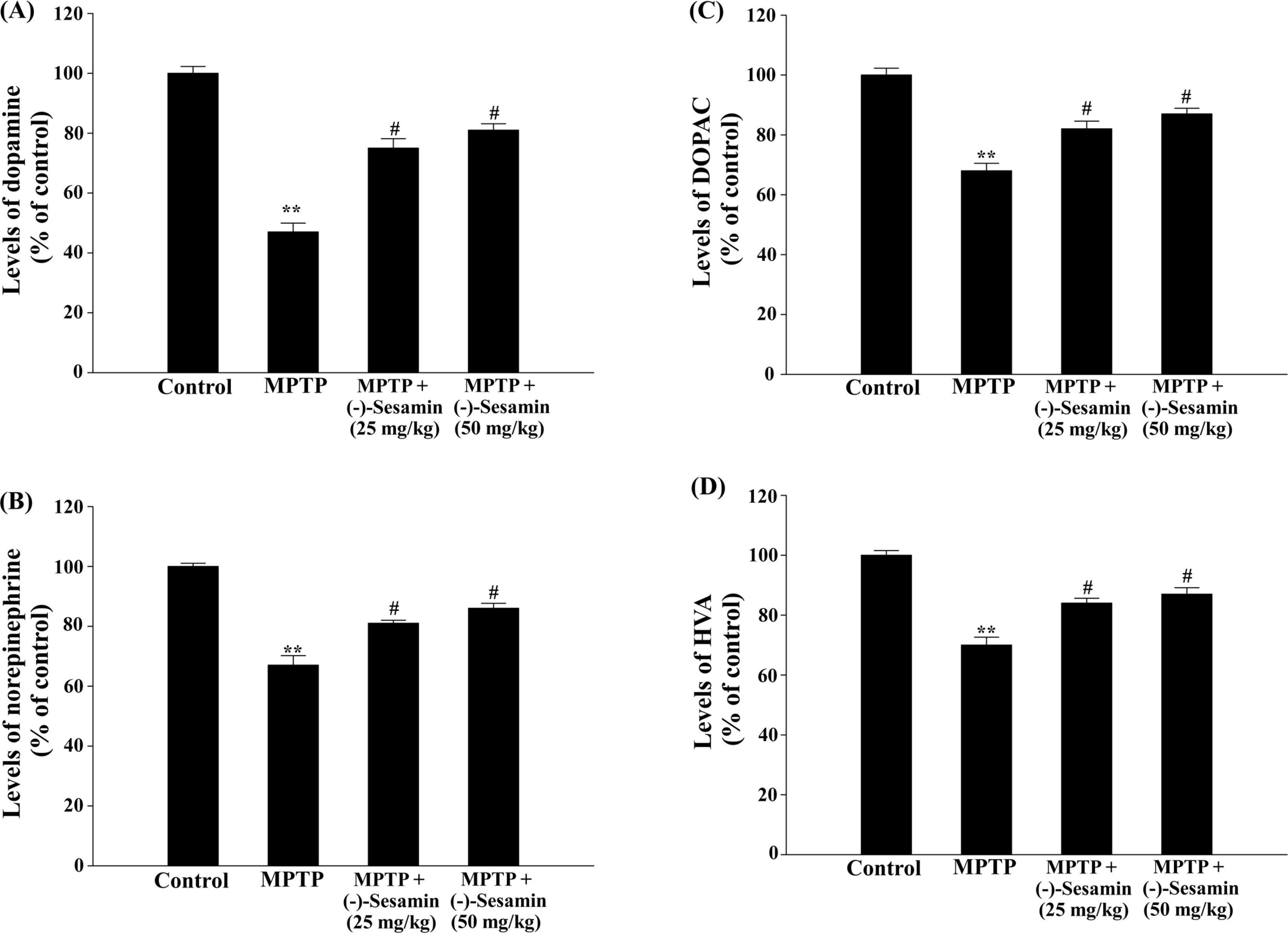
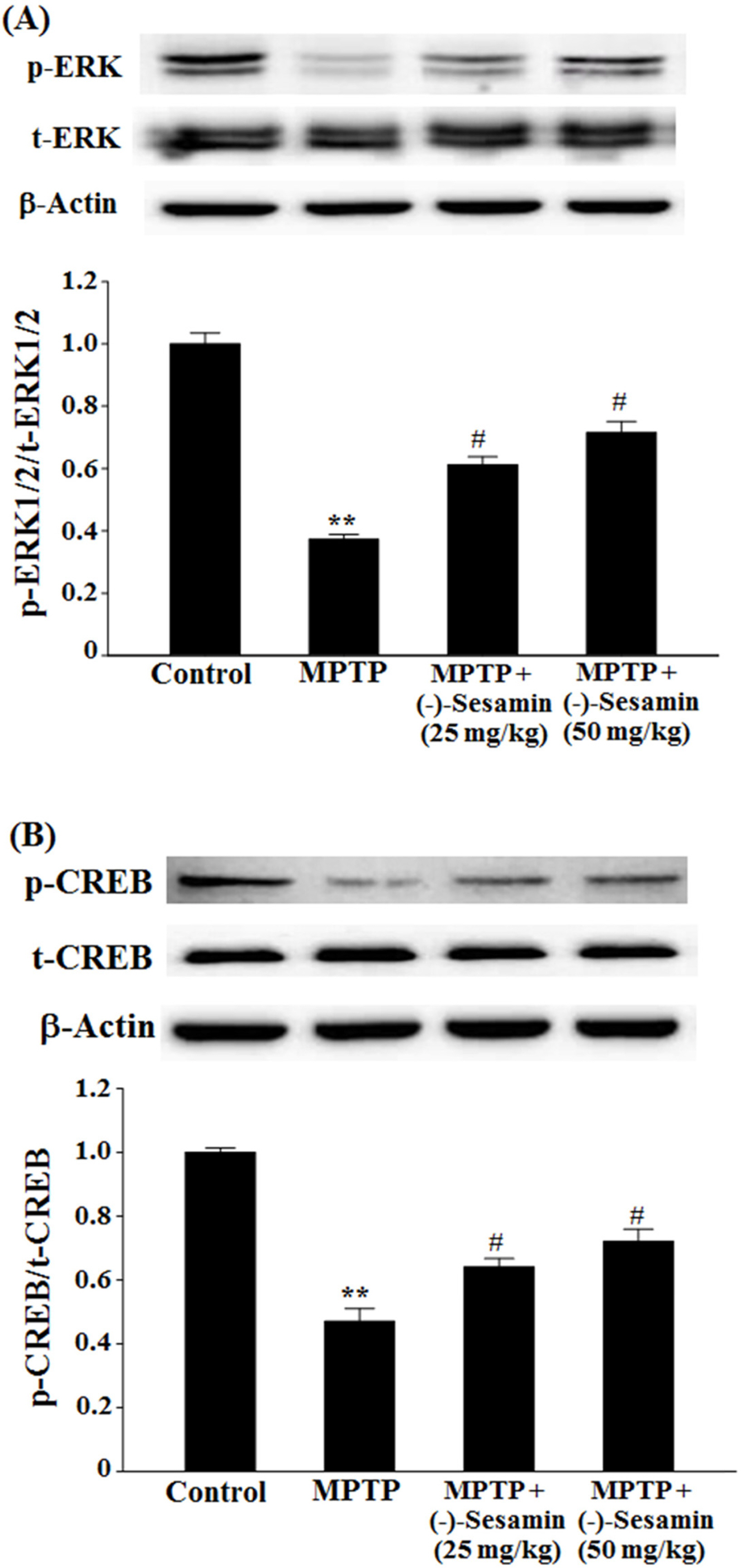
- This study investigated the effects of (−)-sesamin on memory deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse model of Parkinson's disease (PD). MPTP lesion (30 mg/kg/day, 5 days) in mice showed memory deficits including habit learning memory and spatial memory. However, treatment with (−)-sesamin (25 and 50 mg/kg) for 21 days ameliorated memory deficits in MPTP-lesioned mouse model of PD: (−)-sesamin at both doses improved decreases in the retention latency time of the passive avoidance test and the levels of dopamine, norepinephrine, 3,4-dihydroxyphenylacetic acid, and homovanillic acid, improved the decreased transfer latency time of the elevated plus-maze test, reduced the increased expression of N-methyl-D-aspartate (NMDA) receptor, and increased the reduced phosphorylation of extracellular signal-regulated kinase (ERK1/2) and cyclic AMP-response element binding protein (CREB). These results suggest that (−)-sesamin has protective effects on both habit learning memory and spatial memory deficits via the dopaminergic neurons and NMDA receptor-ERK1/2-CREB system in MPTP-lesioned mouse model of PD, respectively. Therefore, (−)-sesamin may serve as an adjuvant phytonutrient for memory deficits in PD patients.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
3,4-Dihydroxyphenylacetic Acid
Animals
Carrier Proteins
Dopamine
Dopaminergic Neurons
Homovanillic Acid
Humans
Learning
Memory Disorders*
Memory*
Mice*
N-Methylaspartate
Norepinephrine
Parkinson Disease*
Phosphorylation
Phosphotransferases
Spatial Memory
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
3,4-Dihydroxyphenylacetic Acid
Carrier Proteins
Dopamine
Homovanillic Acid
N-Methylaspartate
Norepinephrine
Phosphotransferases
Figure
Reference
-
References
(1). Mayeux R.Annu. Rev. Neurosci. 2003. 26:81–104.
Article(2). Buter T. C.., van den Hout A.., Matthews F. E.., Larsen J. P.., Brayne C.., Aarsland D.Neurology. 2008. 70:1017–1022.(3). Williams-Gray C. H.., Foltynie T.., Brayne C. E.., Robbins T. W.., Barker R. A.Brain. 2007. 130:1787–1798.(4). Da Cunha C.., Angelucci M. E. M.., Canteras N. S.., Wonnacott S.., Takahashi R. N.Cell. Mol. Neurobiol. 2002. 22:227–237.
Article(5). Shi L.., Adams M. M.., Long A.., Carter C. C.., Bennett C.., Sonntag W. E.., Nicolle M. M.., Robbins M.., D'Agostino R.., Brunso-Bechtolda J. K.Radiat. Res. 2006. 166:892–899.(6). Wang H. M.., Cheng K. C.., Lin C. J.., Hsu S. W.., Fang W. C.., Hsu T. F.., Chiu C. C.., Chang H. W.., Hsu C. H.., Lee A. Y.Cancer Sci. 2010. 101:2612–2620.(7). Han A. R.., Kim H. J.., Shin M.., Hong M.., Kim Y. S.., Bae H.Chem. Biodivers. 2008. 5:346–351.(8). Peñalvo J. L.., Hopia A.., Adlercreutz H.Eur. J. Nutr. 2006. 45:439–444.(9). Park H. J.., Zhao T. T.., Lee K. S.., Lee S. H.., Shin K. S.., Park K. H.., Choi H. S.., Lee M. K.Neurochem. Int. 2015. 83–84:19–27.(10). Fujikawa T.., Kanada N.., Shimada A.., Ogata M.., Suzuki I.., Hayashi I.., Nakashima K.Biol. Pharm. Bull. 2005. 28:169–172.(11). Zhang M.., Lee H. J.., Park K. H.., Park H. J.., Choi H. S.., Lim S. C.., Lee M. K.Neuropharmacology. 2012. 62:2219–2226.(12). Li C. Y.., Chow T. J.., Wu T. S. J.Nat. Prod. 2005. 68:1622–1624.(13). Schober A.Cell Tissue Res. 2004. 318:215–224.(14). Roghani M.., Baluchnejadnojarad T.Basic Clin. Neurosci. 2010. 1:52–55.(15). Izurieta-Sánchez P.., Sarre S.., Ebinger G.., Michotte Y.Eur. J. Pharmacol. 1998. 353:33–42.(16). Shin K. S.., Choi H. S.., Zhao T. T.., Suh K. H.., Kwon I. H.., Choi S. O.., Lee M. K.Arch. Pharm. Res. 2013. 36:759–767.(17). Kumar B.., Kuhad A.., Chopra K.Psychopharmacology. 2011. 214:819–828.(18). El Massioui N.., Delatour B. V.Neurosci. Res. Comm. 1997. 21:103–111.(19). Matsumoto N.., Hanakawa T.., Maki S.., Graybiel A. M.., Kimura M. J.Neurophysiol. 1999. 82:978–98.(20). Bannerman D. M.., Good M. A.., Butcher S. P.., Ramsay M.., Morris R. G.Nature. 1995. 378:182–186.(21). Krapivinsky G.., Krapivinsky L.., Manasian L.., Ivanov A.., Tyzio R.., Pellegrino C.., Ben-Ari Y.., Clapham D. E.., Medina I.Neuron. 2003. 40:775–784.(22). Carlezon Jr W. A.., Duman R. S.., Nestler E. J.Trends Neurosci. 2005. 28:436–445.(23). Heikkila R. E.., Hess A.., Duvoisin R. C.Science. 1984. 224:1451–1453.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on Motor Behavior and Dopamine Levels at Brain Regions in Three Different Mouse Strains
- Sinapic Acid Ameliorates REV-ERB α Modulated Mitochondrial Fission against MPTP-Induced Parkinson’s Disease Model
- Ameliorative Effects of NXP031 on MPTP-Induced Neurotoxicity
- Behavioral and Histochemical Changes in MPTP-treated C57BL/6 Mice: A Model for Parkinson's Disease
- Comparison of the Effects of NOS Inhibitor or HS-1580 on Nigrostriatal Dopaminergic System in MPTP-induced Parkinson's Disease Animal Model