Int J Stem Cells.
2016 Nov;9(2):213-220. 10.15283/ijsc16031.
Impact of Cell Density on Differentiation Efficiency of Rat Adipose-derived Stem Cells into Schwann-like Cells
- Affiliations
-
- 1Cellular and Molecular Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. bayati-v@ajums.ac.ir vahid_bayati@yahoo.com
- 2Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- KMID: 2362515
- DOI: http://doi.org/10.15283/ijsc16031
Abstract
- BACKGROUND AND OBJECTIVES
Schwann-like (SC-like) cells induced from adipose-derived stem cells (ASCs) may be one of the ideal alternative cell sources for obtaining Schwann cells (SCs). They can be used for treating peripheral nerve injuries. Co-culture with SCs or exposure to glial growth factors are commonly used for differentiation of ASCs to SC-like cells. However, the effect of initial cell density as an inductive factor on the differentiation potential of ASCs into the SC-like cells has not been yet investigated.
METHODS AND RESULTS
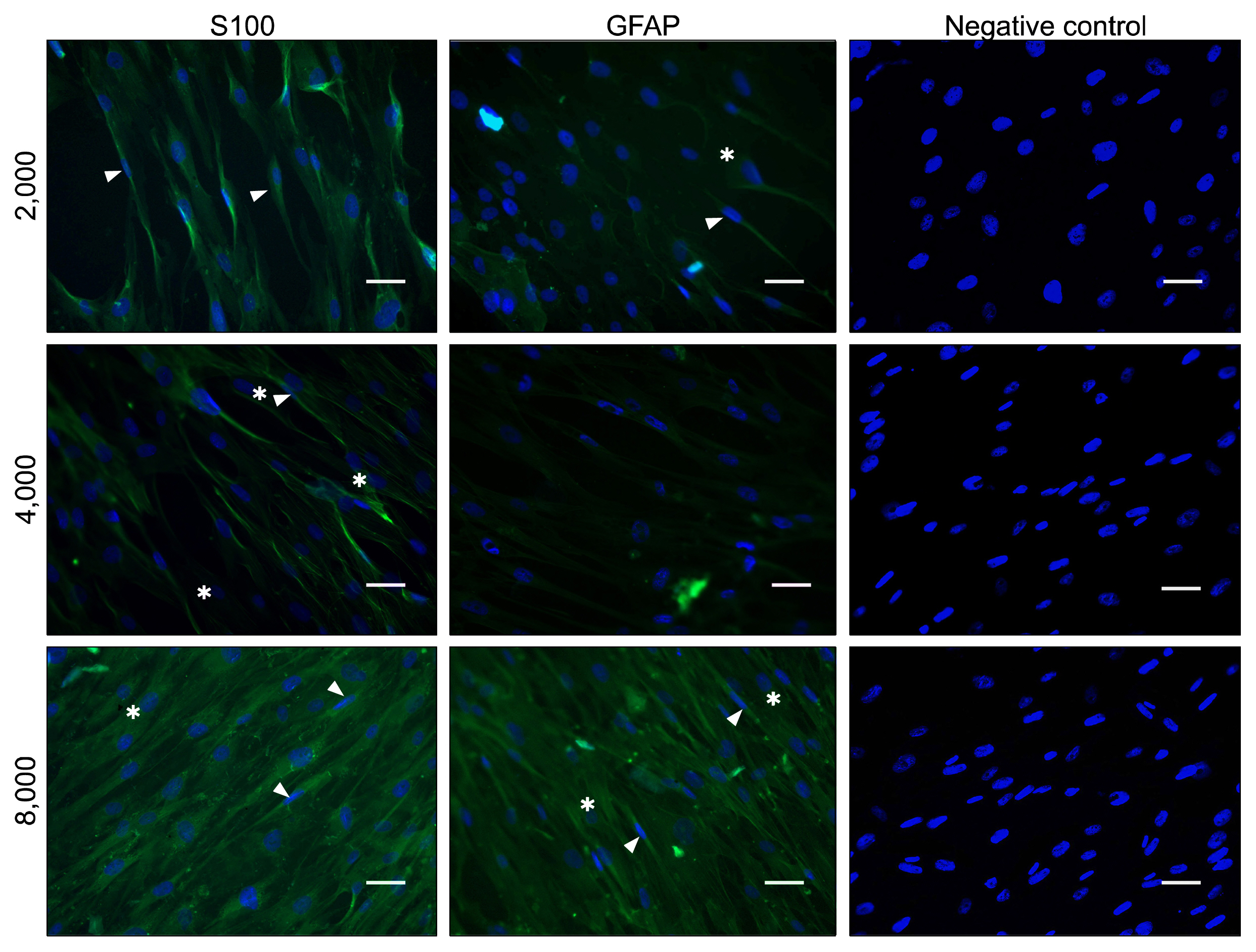
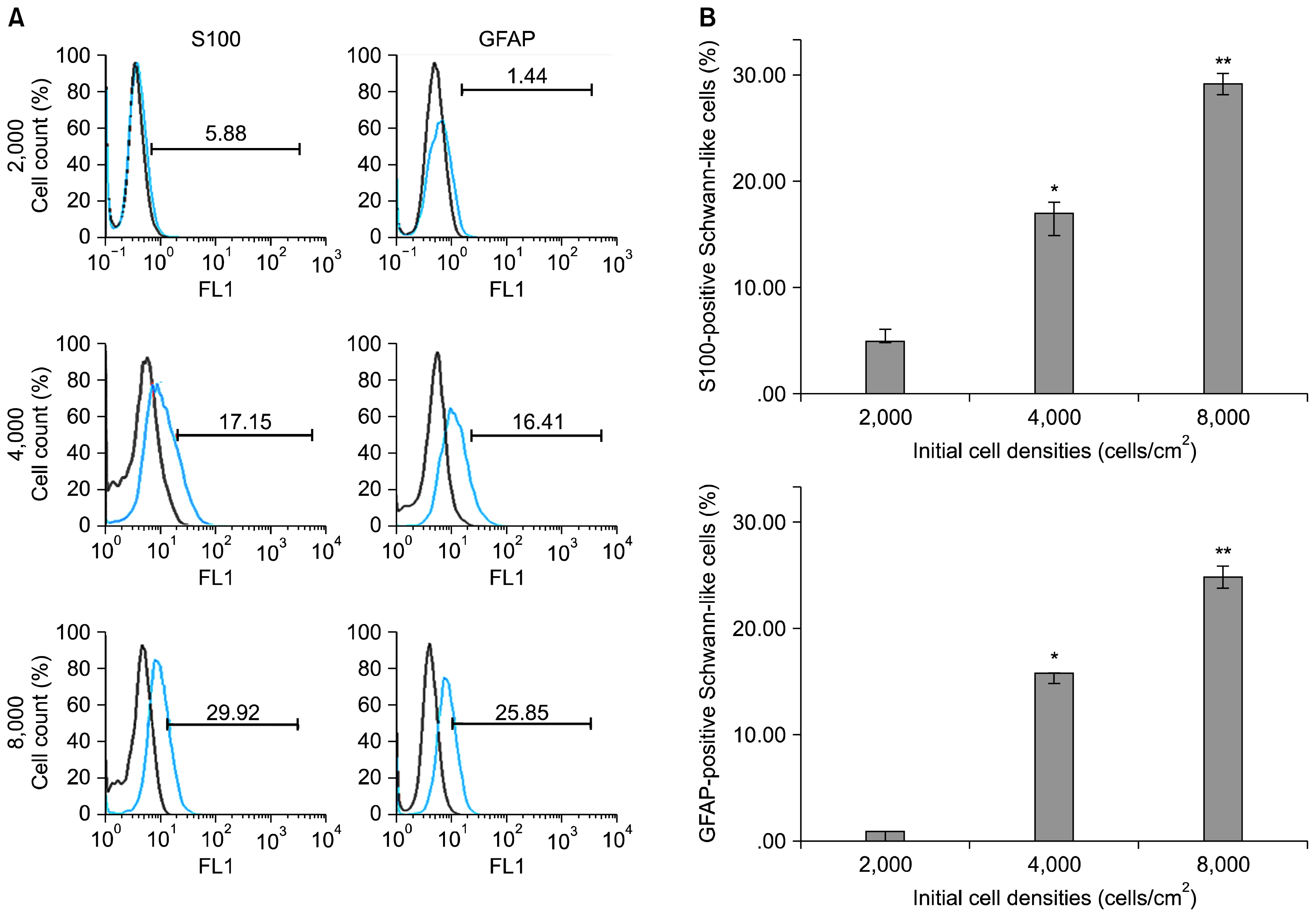
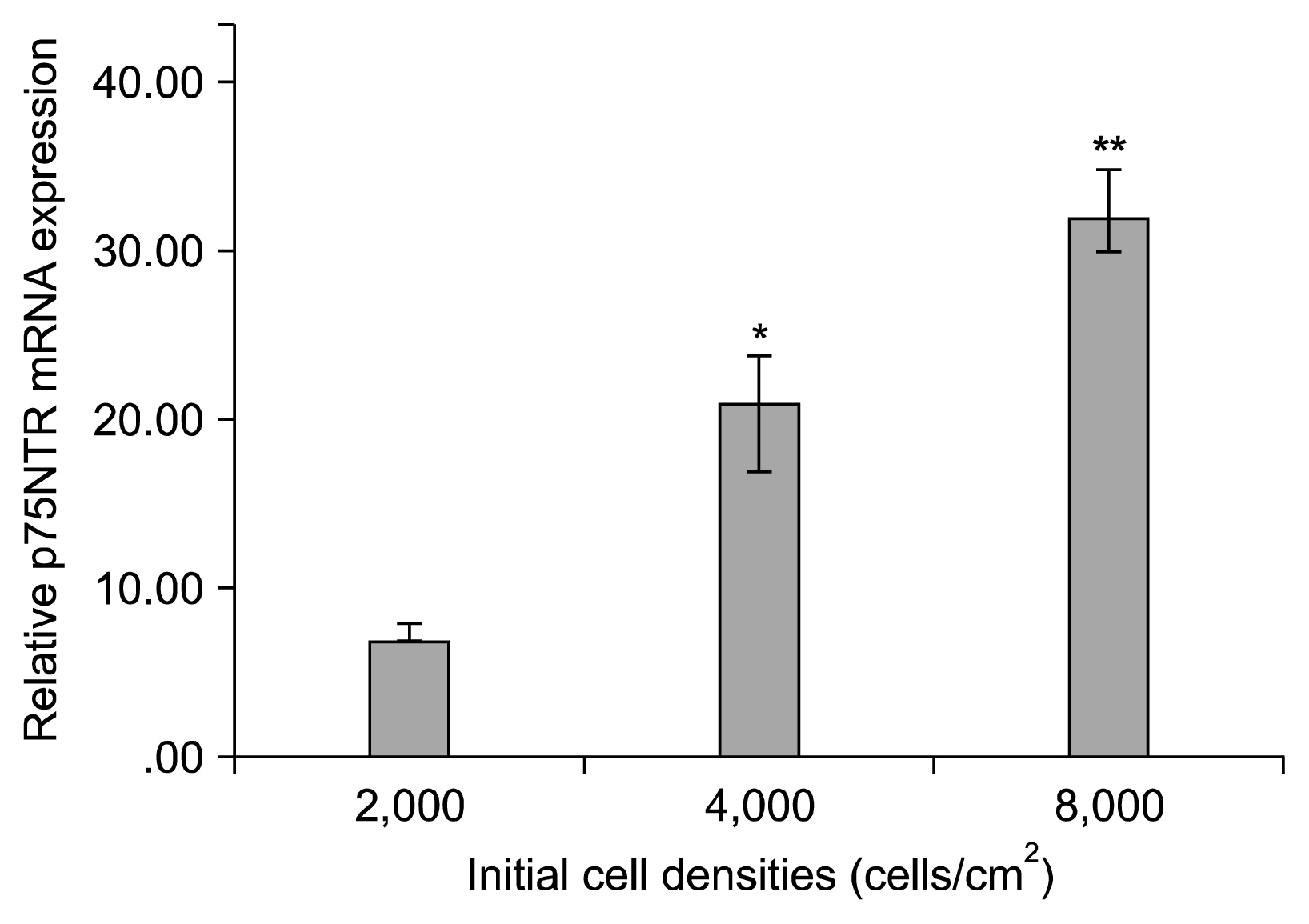
ASCs were harvested from rat and characterized. The cells were seeded into the culture flasks at three different initial cell densities i.e. 2×10³, 4×10³ and 8×10³ cells/cm² an overnight and differentiated toward SC-like cells using glial growth factors. After two weeks, the differentiation rate of ASCs to SC-like cells at different densities was assessed by immunofluorescence, fluorescence-activated cell sorting analysis and real time RT-PCR. Expression of the typical SCs markers, S-100 proteins and glial fibrillary acidic protein (GFAP) protein, was observed in all cell densities groups although the number of S100-positive and GFAP-positive cells, and the expression of p75(NTR) mRNA, another SC marker, were significantly higher at the density of 8×10³ cells/cm² when compared with the other cell densities groups (p<0.001).
CONCLUSIONS
The results suggest that the higher differentiation rate of ASCs to SC-like cells can be obtained at initial cell density of 8×10³ cells/cm², possibly via increased cell-cell interaction and cell density-dependent influence of glial growth factors.
MeSH Terms
Figure
Reference
-
References
1. Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surg Technol Int. 2003; 11:303–310. PMID: 12931315.2. Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z, Gu R, Hou X, Zhang C. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008; 1239:49–55. DOI: 10.1016/j.brainres.2008.08.088. PMID: 18804456.
Article3. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260. DOI: 10.1161/01.RES.0000265074.83288.09. PMID: 17495232.
Article4. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295. DOI: 10.1091/mbc.E02-02-0105. PMID: 12475952. PMCID: 138633.
Article5. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001; 24:1217–1281. DOI: 10.1146/annurev.neuro.24.1.1217. PMID: 11520933.
Article6. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007; 207:267–274. DOI: 10.1016/j.expneurol.2007.06.029. PMID: 17761164.
Article7. Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006; 74:510–518. DOI: 10.1111/j.1432-0436.2006.00081.x. PMID: 17177848.
Article8. Pourheydar B, Joghataei MT, Bakhtiari M, Mehdizadeh M, Yekta Z, Najafzadeh N. Co- transplantation of bone marrow stromal cells with schwann cells evokes mechanical allodynia in the contusion model of spinal cord injury in rats. Cell J. 2012; 13:213–222. PMID: 23508042. PMCID: 3584477.9. Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013; 236:55–65. DOI: 10.1016/j.neuroscience.2012.12.066. PMID: 23370324.
Article10. Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005; 40:229–242. DOI: 10.1080/10409230591008189. PMID: 16126487.
Article11. Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007; 6:385–389. DOI: 10.4161/cc.6.4.3804. PMID: 17314508.
Article12. Bayati V, Sadeghi Y, Shokrgozar MA, Haghighipour N, Azadmanesh K, Amanzadeh A, Azari S. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell. 2011; 43:359–366. DOI: 10.1016/j.tice.2011.07.004. PMID: 21872289.
Article13. Guo L, Kawazoe N, Fan Y, Ito Y, Tanaka J, Tateishi T, Zhang X, Chen G. Chondrogenic differentiation of human mesenchymal stem cells on photoreactive polymer-modified surfaces. Biomaterials. 2008; 29:23–32. DOI: 10.1016/j.biomaterials.2007.08.043.
Article14. Song SJ, Jeon O, Yang HS, Han DK, Kim BS. Effects of culture conditions on osteogenic differentiation in human mesenchymal stem cells. J Microbiol Biotechnol. 2007; 17:1113–1119. PMID: 18051321.15. Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005; 33:943–950. DOI: 10.1007/s10439-005-3311-8. PMID: 16060535.
Article16. Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009; 15:1009–1017. DOI: 10.1089/ten.tea.2008.0012.
Article17. Goldstein AS. Effect of seeding osteoprogenitor cells as dense clusters on cell growth and differentiation. Tissue Eng. 2001; 7:817–827. DOI: 10.1089/107632701753337753. PMID: 11749737.
Article18. Bitar M, Brown RA, Salih V, Kidane AG, Knowles JC, Nazhat SN. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules. 2008; 9:129–135. DOI: 10.1021/bm701112w.
Article19. Kruyt M, De Bruijn J, Rouwkema J, Van Bliterswijk C, Oner C, Verbout A, Dhert W. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic cells in cell-based bone tissue engineering in goats. Tissue Eng Part A. 2008; 14:1081–1088. DOI: 10.1089/ten.tea.2007.0111. PMID: 18558815.
Article20. Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly (propylene fumarate) disks. Biomacromolecules. 2009; 10:1810–1817. DOI: 10.1021/bm900240k. PMID: 19469498. PMCID: 3655530.
Article21. Xu HH, Simon CG Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005; 26:1337–1348. DOI: 10.1016/j.biomaterials.2004.04.043.
Article22. Takagi M, Umetsu Y, Fujiwara M, Wakitani S. High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J Biosci Bioeng. 2007; 103:98–100. DOI: 10.1263/jbb.103.98. PMID: 17298908.
Article23. Yassin MA, Leknes KN, Pedersen TO, Xing Z, Sun Y, Lie SA, Finne-Wistrand A, Mustafa K. Cell seeding density is a critical determinant for copolymer scaffolds-induced bone regeneration. J Biomed Mater Res A. 2015; 103:3649–3658. DOI: 10.1002/jbm.a.35505. PMID: 26013960. PMCID: 4744655.
Article24. Maia FR, Lourenço AH, Granja PL, Gonçalves RM, Barrias CC. Effect of cell density on mesenchymal stem cells aggregation in RGD-alginate 3D matrices under osteoinductive conditions. Macromol Biosci. 2014; 14:759–771. DOI: 10.1002/mabi.201300567. PMID: 24585449.
Article25. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004; 6:483–495. DOI: 10.1016/S1534-5807(04)00075-9. PMID: 15068789.
Article26. Both SK, van der Muijsenberg AJ, van Blitterswijk CA, de Boer J, de Bruijn JD. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007; 13:3–9. DOI: 10.1089/ten.2005.0513. PMID: 17518576.
Article27. Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000; 97:3213–3218. DOI: 10.1073/pnas.97.7.3213. PMID: 10725391. PMCID: 16218.
Article28. Younesi E, Bayati V, Hashemitabar M, Azandeh SS, Bijannejad D, Bahreini A. Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum? Anat Cell Biol. 2015; 48:170–176. DOI: 10.5115/acb.2015.48.3.170. PMID: 26417476. PMCID: 4582159.
Article29. Radtke C, Schmitz B, Spies M, Kocsis JD, Vogt PM. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int J Dev Neurosci. 2009; 27:817–823. DOI: 10.1016/j.ijdevneu.2009.08.006. PMID: 19699793.
Article30. Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991; 112:457–467. DOI: 10.1083/jcb.112.3.457. PMID: 1704008. PMCID: 2288828.
Article31. Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J Neurosci. 2001; 21:1110–1116. PMID: 11160381.
Article32. Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998; 21:681–693. DOI: 10.1016/S0896-6273(00)80586-3. PMID: 9808456. PMCID: 2693071.
Article33. Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997; 49:236–247. DOI: 10.1002/(SICI)1097-4547(19970715)49:2<236::AID-JNR12>3.0.CO;2-Z. PMID: 9272646.
Article34. Zaminy A, Shokrgozar MA, Sadeghi Y, Noroozian M, Heidari MH, Piryaei A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran Biomed J. 2013; 17:113–122. PMID: 23748888. PMCID: 3770252.35. Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995; 15:585–596. DOI: 10.1016/0896-6273(95)90147-7. PMID: 7546738.
Article36. Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006; 16:492–500. DOI: 10.1016/j.conb.2006.08.008. PMID: 16962312.
Article37. Levi AD, Bunge RP, Lofgren JA, Meima L, Hefti F, Nikolics K, Sliwkowski MX. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995; 15:1329–1340. PMID: 7869101.
Article38. Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996; 17:327–338. DOI: 10.1002/(SICI)1098-1136(199608)17:4<327::AID-GLIA7>3.0.CO;2-W. PMID: 8856329.
Article39. Casella GT, Wieser R, Bunge RP, Margitich IS, Katz J, Olson L, Wood PM. Density dependent regulation of human Schwann cell proliferation. Glia. 2000; 30:165–177. DOI: 10.1002/(SICI)1098-1136(200004)30:2<165::AID-GLIA6>3.0.CO;2-L. PMID: 10719358.
Article40. Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997; 36:17–28. DOI: 10.1002/(SICI)1097-4636(199707)36:1<17::AID-JBM3>3.0.CO;2-O. PMID: 9212385.
Article41. Toh YC, Ho ST, Zhou Y, Hutmacher DW, Yu H. Application of a polyelectrolyte complex coacervation method to improve seeding efficiency of bone marrow stromal cells in a 3D culture system. Biomaterials. 2005; 26:4149–4160. DOI: 10.1016/j.biomaterials.2004.10.033. PMID: 15664642.
Article42. Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011; 32:8927–8937. DOI: 10.1016/j.biomaterials.2011.08.027. PMID: 21906805. PMCID: 3183393.
Article43. Banoub RW, Fernstrom M, Ruch RJ. Lack of growth inhibition or enhancement of gap junctional intercellular communication and connexin43 expression by beta-carotene in murine lung epithelial cells in vitro. Cancer Lett. 1996; 108:35–40. DOI: 10.1016/S0304-3835(96)04367-4. PMID: 8950206.
Article44. van den Dolder J, Spauwen PH, Jansen JA. Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng. 2003; 9:315–325. DOI: 10.1089/107632703764664783. PMID: 12740094.
Article45. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002; 20:530–541. DOI: 10.1634/stemcells.20-6-530. PMID: 12456961.
Article46. Zhou H, Weir MD, Xu HH. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement-fiber scaffold. Tissue Eng Part A. 2011; 17:2603–2613. DOI: 10.1089/ten.tea.2011.0048. PMID: 21745111. PMCID: 3204200.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- Adipose-derived stem cells: characterization and clinical application
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study