Anat Cell Biol.
2015 Sep;48(3):170-176. 10.5115/acb.2015.48.3.170.
Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- Affiliations
-
- 1Cellular and Molecular Research Center, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. vahid_bayati@yahoo.com
- 2Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 3Transplant Ward, Ahvaz Golestan Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- KMID: 2424819
- DOI: http://doi.org/10.5115/acb.2015.48.3.170
Abstract
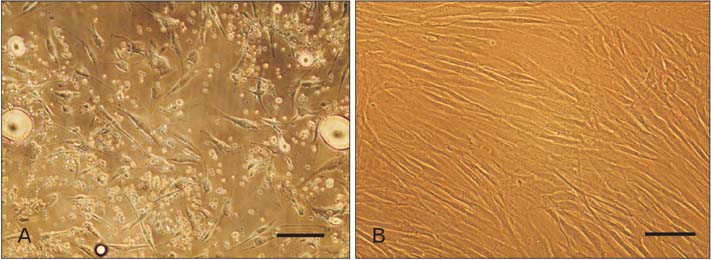
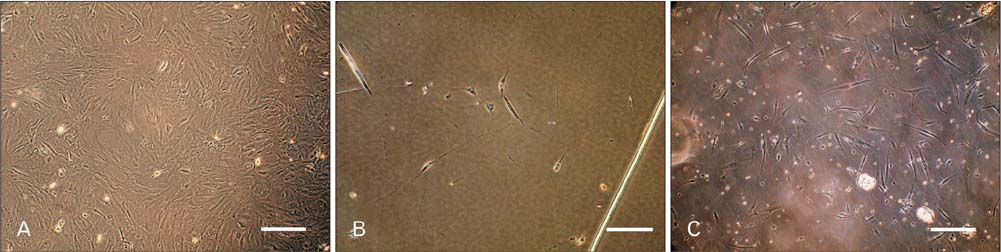
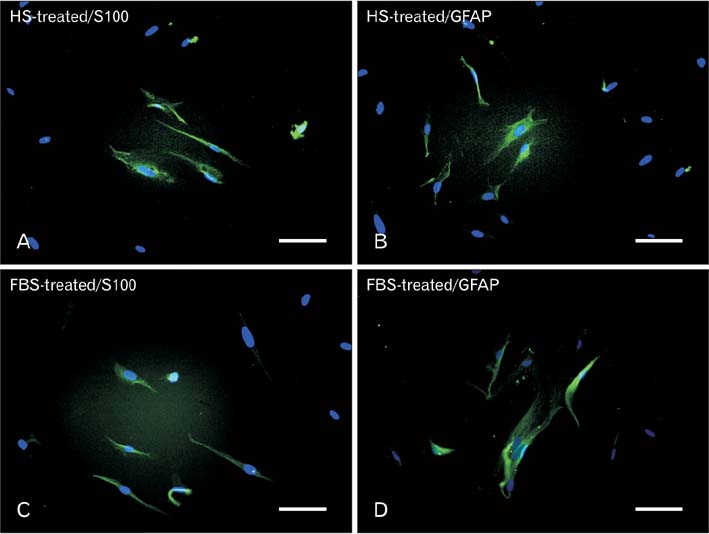
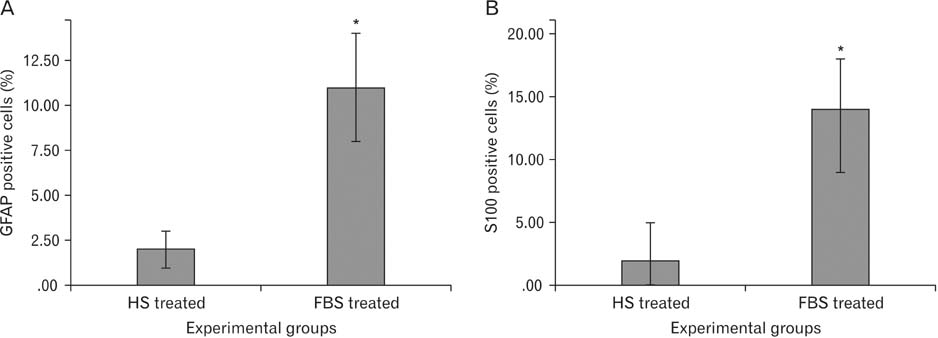
- Access to autologous Schwann cells is limited due to lack of donor site and its difficult isolation and culture. Therefore, one of the possible ways to obtain to Schwann cells is to differentiate mesenchymal stem cells into glial pathway using various materials and protocols. The aim of this study was to compare the effects of fetal bovine serum and human serum on Schwann cell differentiation of adipose-derived stem cells to choose the best serum for use in future research. For this purpose, after isolation of human adipose-derived stem cells, it was characterized and differentiated into Schwann cell lineage using two protocols which one of them contained fetal bovine serum and the other human serum. At the end, morphological evaluation declared an increased detachment of cells in response to human serum. On the other side, immunocytochemistry showed that there was a significant increase in the number of cells expressing glial fibrillary acidic proteins and S100 in fetal bovine serum-treated group when compared to human serum-treated one (P<0.05). It was concluded that fetal bovine serum was more effective than allogeneic human serum in Schwann cell differentiation of adipose-derived stem cells.
MeSH Terms
Figure
Cited by 2 articles
-
Expression of neurotrophic factor genes by human adipose stem cells post-induction by deprenyl
Arezoo Amiri, Maryam Haji Ghasem Kashani, Mohammad Taghi Ghorbanian
Anat Cell Biol. 2021;54(1):74-82. doi: 10.5115/acb.19.229.Impact of Cell Density on Differentiation Efficiency of Rat Adipose-derived Stem Cells into Schwann-like Cells
Mahtab Maghzi Najafabadi, Vahid Bayati, Mahmoud Orazizadeh, Mahmoud Hashemitabar, Forouzan Absalan
Int J Stem Cells. 2016;9(2):213-220. doi: 10.15283/ijsc16031.
Reference
-
1. Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013; 236:55–65.2. Jung J, Cai W, Jang SY, Shin YK, Suh DJ, Kim JK, Park HT. Transient lysosomal activation is essential for p75 nerve growth factor receptor expression in myelinated Schwann cells during Wallerian degeneration. Anat Cell Biol. 2011; 44:41–49.3. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007; 207:267–274.4. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003; 5:362–369.5. Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010; 42:211–216.6. di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010; 63:1544–1552.7. Tomita K, Madura T, Mantovani C, Terenghi G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. 2012; 90:1392–1402.8. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004; 8:301–316.9. Erfanmanesh A, Gholampuor H, Gharibi S. The effect of different doses of gamma irradiation on the sterilization of fetal bovine serum. J Microb Biotechnol. 2011; 3:1–6.10. Russell KA, Koch TG. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture: too much of a good thing? Equine Vet J. 2015; 03. 16. DOI: 10.1111/evj.12440. [Epub].11. Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000; 49:152–156.12. Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997; 89:776–779.13. Tuschong L, Soenen SL, Blaese RM, Candotti F, Muul LM. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002; 13:1605–1610.14. Sasse M, Lengwinat T, Henklein P, Hlinak A, Schade R. Replacement of fetal calf serum in cell cultures by an egg yolk factor with cholecystokinin/gastrin-like immunoreactivity. Altern Lab Anim. 2000; 28:815–831.15. Schwartz KA, Lu G, Trosko JE, Chang CC. Serum from outdated human platelet concentrates: an alternative supplement for tissue (fibroblast) culture media. Am J Hematol. 1984; 17:23–27.16. Clemmons DR, Isley WL, Brown MT. Dialyzable factor in human serum of platelet origin stimulates endothelial cell replication and growth. Proc Natl Acad Sci U S A. 1983; 80:1641–1645.17. Pietschmann P, Stöckl J, Draxler S, Majdic O, Knapp W. Functional and phenotypic characteristics of dendritic cells generated in human plasma supplemented medium. Scand J Immunol. 2000; 51:377–383.18. Filipic B, Shehata M, Toth S, Schwarzmeier J, Koren S. Novel serum replacement based on bovine ocular fluid: a useful tool for cultivation of different animal cells in vitro. ALTEX. 2002; 19:15–20.19. Grace S, Guthrie LA, Johnston RB Jr. The use of mouse serum and the presence of non-adherent cells for the culture of mouse macrophages. J Immunol Methods. 1988; 114:21–26.20. Reisser D, Fady C, Pelletier H, Lagadec P, Jeannin JF, Olsson NO. Comparative effect of rat and fetal calf serum on measurement of the natural tumoricidal activity of rat lymphocytes, macrophages and polymorphonuclear cells. Cancer Immunol Immunother. 1989; 28:34–36.21. Mangalo R, Marcovich H. Mitogenic factors for BALB/c 3T3 cells isolated from the serum of horses by affinity chromatography on a column using fetal calf serum as the ligand. C R Acad Sci III. 1984; 299:445–450.22. Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005; 23:1357–1366.23. Kobayashi T, Watanabe H, Yanagawa T, Tsutsumi S, Kayakabe M, Shinozaki T, Higuchi H, Takagishi K. Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. J Bone Joint Surg Br. 2005; 87:1426–1433.24. Choi J, Chung JH, Kwon GY, Kim KW, Kim S, Chang H. Effectiveness of autologous serum as an alternative to fetal bovine serum in adipose-derived stem cell engineering. Cell Tissue Bank. 2013; 14:413–422.25. Bieback K, Hecker A, Schlechter T, Hofmann I, Brousos N, Redmer T, Besser D, Klüter H, Müller AM, Becker M. Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy. 2012; 14:570–583.26. Kocaoemer A, Kern S, Klüter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007; 25:1270–1278.27. Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol. 2004; 32:1212–1225.28. Oreffo RO, Virdi AS, Triffitt JT. Modulation of osteogenesis and adipogenesis by human serum in human bone marrow cultures. Eur J Cell Biol. 1997; 74:251–261.29. Yamamoto N, Isobe M, Negishi A, Yoshimasu H, Shimokawa H, Ohya K, Amagasa T, Kasugai S. Effects of autologous serum on osteoblastic differentiation in human bone marrow cells. J Med Dent Sci. 2003; 50:63–69.30. Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008; 58:501–510.31. Tateishi K, Ando W, Higuchi C, Hart DA, Hashimoto J, Nakata K, Yoshikawa H, Nakamura N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical applications. Cell Transplant. 2008; 17:549–557.32. Pisciotta A, Riccio M, Carnevale G, Beretti F, Gibellini L, Maraldi T, Cavallini GM, Ferrari A, Bruzzesi G, De Pol A. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS One. 2012; 7:e50542.33. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. 2006; 24:1409–1410.34. Smith SR, Munavalli G, Weiss R, Maslowski JM, Hennegan KP, Novak JM. A multicenter, double-blind, placebo-controlled trial of autologous fibroblast therapy for the treatment of nasolabial fold wrinkles. Dermatol Surg. 2012; 38(7 Pt 2):1234–1243.35. Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013; 382:1113–1120.36. Chhetri DK, Berke GS. Injection of cultured autologous fibroblasts for human vocal fold scars. Laryngoscope. 2011; 121:785–792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media
- Impact of Cell Density on Differentiation Efficiency of Rat Adipose-derived Stem Cells into Schwann-like Cells
- The Effects of Human Bone Marrow-Derived Mesenchymal Stem Cell Conditioned Media Produced with Fetal Bovine Serum or Human Platelet Lysate on Skin Rejuvenation Characteristics