Int J Stem Cells.
2017 Nov;10(2):184-192. 10.15283/ijsc17028.
A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Affiliations
-
- 1Department of Microbiology and Biochemistry, Faculty of Pharmacy, Damascus University, Damascus, Syria. hsn.ghmkin@gmail.com maljamali@gmail.com
- 2Department of Animal Biology, Faculty of Sciences, Damascus University, Damascus, Syria.
- 3National Commission for Biotechnology (NCBT), Damascus, Syria.
- KMID: 2400873
- DOI: http://doi.org/10.15283/ijsc17028
Abstract
- BACKGROUND AND OBJECTIVES
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be isolated from umbilical cords and are therapeutically used because of their ability to differentiate into various types of cells, in addition to their immunosuppressive and anti-inflammatory properties. Fetal bovine serum (FBS), considered as the standard additive when isolating and culturing MSCs, has a major limitation related to its animal origin. Here, we employed a simple and economically efficient protocol to isolate MSCs from human umbilical cord tissues without using digestive enzymes and replacing FBS with umbilical cord blood serum (CBS).
METHODS AND RESULTS
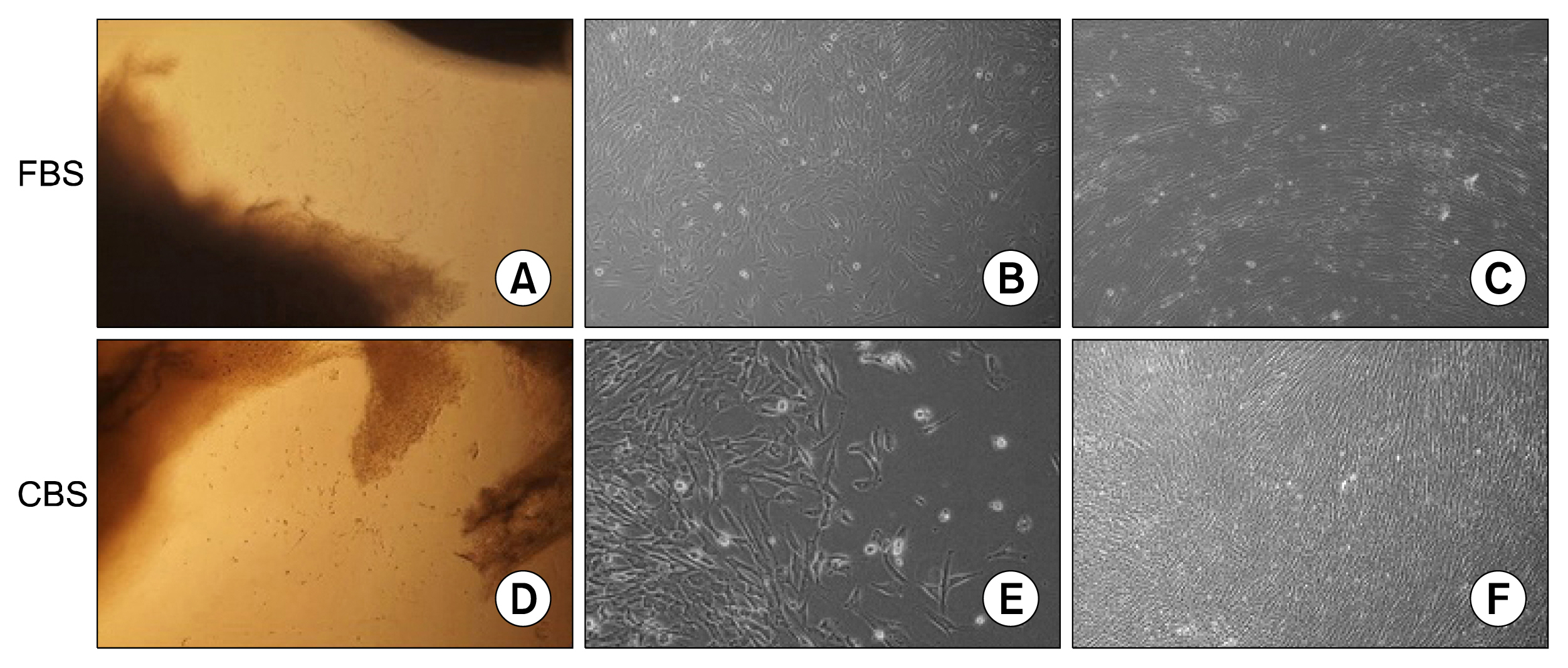
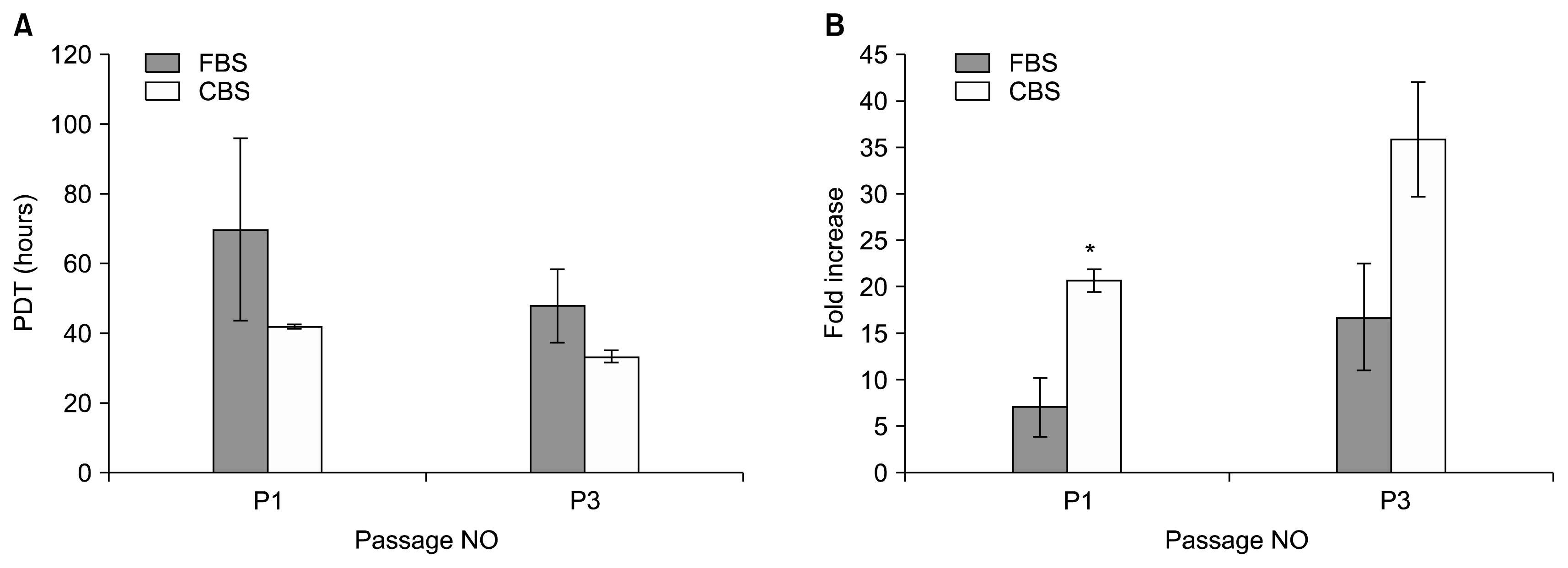
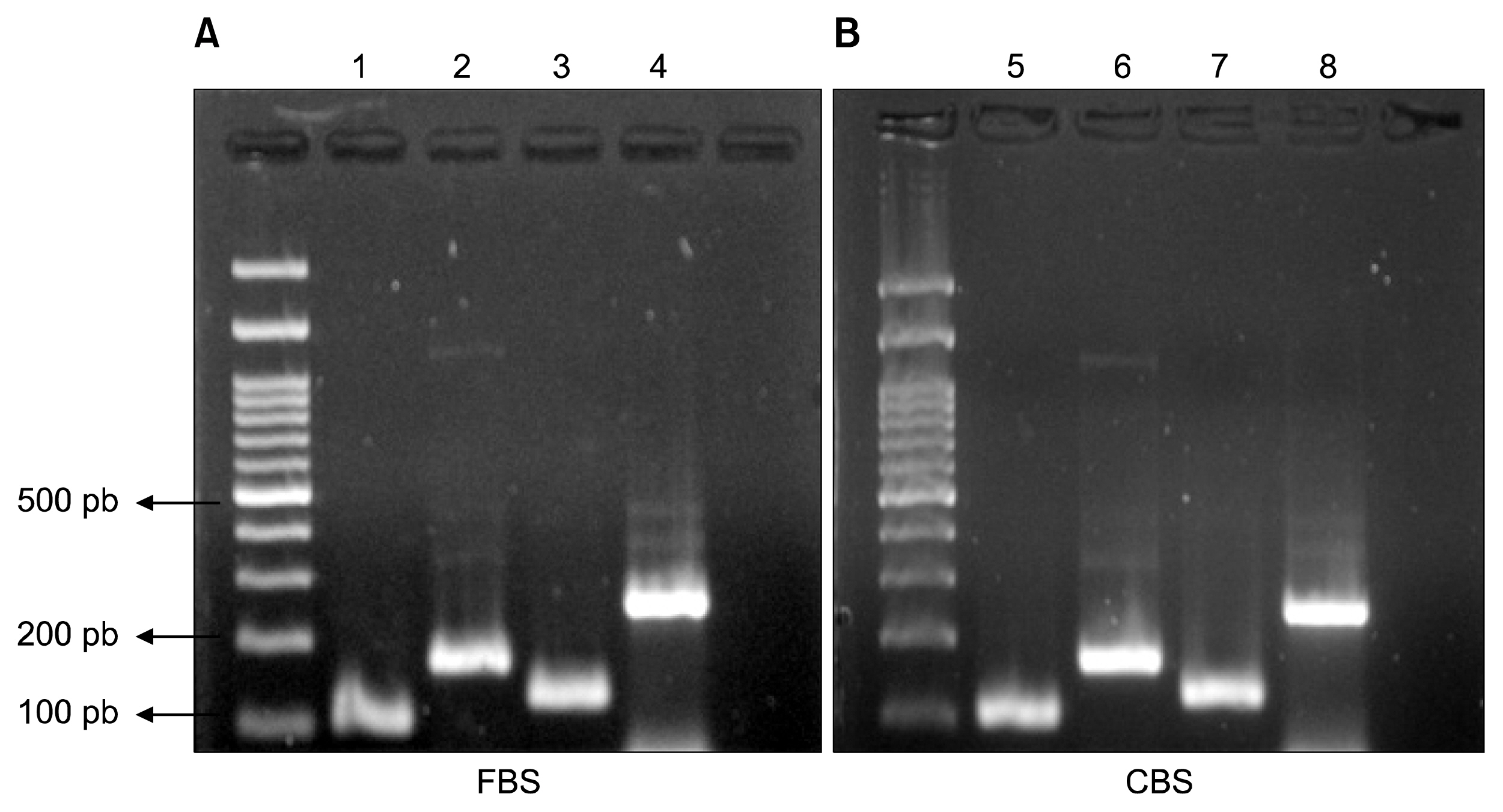
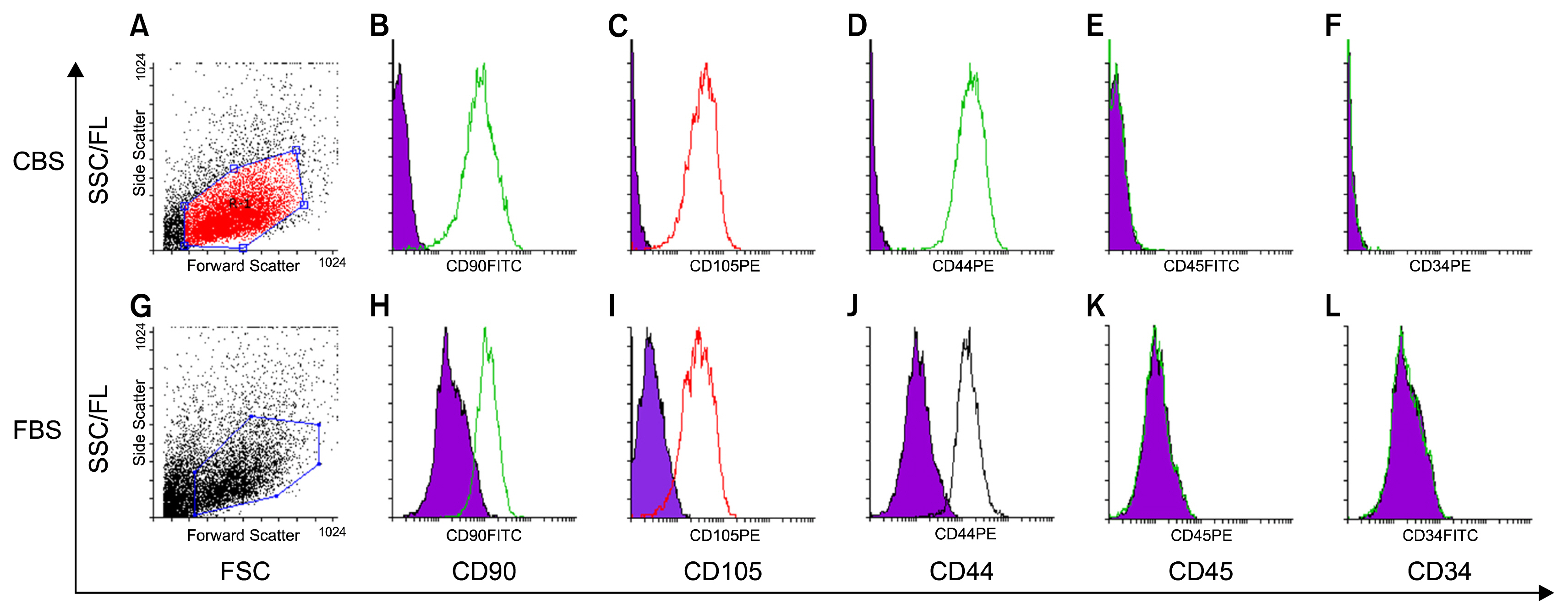
MSCs were isolated by culturing umbilical cord pieces in CBS or FBS supplemented media. Expansion and proliferation kinetics of cells isolated by explant method in the presence of either FBS or CBS were measured, with morphology and multi-differentiation potential of expanded cells characterized by flow cytometry, RT-PCR, and immunofluorescence. MSCs maintained morphology, immunophenotyping, multi-differentiation potential, and self-renewal ability, with better proliferation rates for cells cultured in CBS compared to FBS supplement media.
CONCLUSIONS
We here present a simple, reliable and efficient method to isolate MSCs from umbilical cord tissues, where cells maintained proliferation, differentiation potential and immunophenotyping properties and could be efficiently expanded for clinical applications.
MeSH Terms
Figure
Cited by 2 articles
-
Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
Hussein Baharlooi, Maryam Azimi, Zahra Salehi, Maryam Izad
Int J Stem Cells. 2019;13(1):13-23. doi: 10.15283/ijsc19108.Characterization and Differentiation of Circulating Blood Mesenchymal Stem Cells and the Role of Phosphatidylinositol 3-Kinase in Modulating the Adhesion
Yoon-Kyung Park, Seong-Joo Heo, Jai-Young Koak, Gang-Seok Park, Tae-Jun Cho, Seong-Kyun Kim, Jaejin Cho
Int J Stem Cells. 2019;12(2):265-278. doi: 10.15283/ijsc18136.
Reference
-
References
1. Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006; 2:155–162. DOI: 10.1007/s12015-006-0022-y.
Article2. Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007; 25:2886–2895. DOI: 10.1634/stemcells.2007-0417. PMID: 17690177.
Article3. Chua SJ, Bielecki R, Wong CJ, Yamanaka N, Rogers IM, Casper RF. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feeder-free cell culture. Biochem Biophys Res Commun. 2009; 379:217–221. DOI: 10.1016/j.bbrc.2008.12.045.
Article4. Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015; 24:339–347. DOI: 10.3727/096368915X686841. PMID: 25622293.
Article5. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013; 34:747–754. DOI: 10.1038/aps.2013.50. PMID: 23736003. PMCID: 4002895.
Article6. Cui R, Rekasi H, Hepner-Schefczyk M, Fessmann K, Petri RM, Bruderek K, Brandau S, Jäger M, Flohé SB. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther. 2016; 7:88. DOI: 10.1186/s13287-016-0353-9. PMID: 27388156. PMCID: 4937587.
Article7. Fierabracci A, Del Fattore A, Muraca M. The immunoregulatory activity of mesenchymal stem cells: ‘state of art’ and ‘future avenues’. Curr Med Chem. 2016; 23:3014–3024. DOI: 10.2174/0929867323666160627112827.
Article8. Helal MA, Shaheen NE, Abu Zahra FA. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol Immunotoxicol. 2016; 1–9. PMID: 27560658.
Article9. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004; 103:1669–1675. DOI: 10.1182/blood-2003-05-1670.
Article10. Wolfe M, Pochampally R, Swaney W, Reger RL. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs). Methods Mol Biol. 2008; 449:3–25. PMID: 18370080.
Article11. Avola R, Graziano AC, Pannuzzo G, Cardile V. Human mesenchymal stem cells from adipose tissue differentiated into neuronal or glial phenotype express different aqua-porins. Mol Neurobiol. 2016; [Epub ahead of print].
Article12. Ou H, Zhao S, Peng Y, Xiao X, Wang Q, Liu H, Xiao X, Yang M. Comparison of bone marrow tissue- and adipose tissue-derived mesenchymal stem cells in the treatment of sepsis in a murine model of lipopolysaccharide-induced sepsis. Mol Med Rep. 2016; 14:3862–3870. DOI: 10.3892/mmr.2016.5694. PMID: 27600821.
Article13. Ding Y, Lu Z, Yuan Y, Wang X, Li D, Zeng Y. Comparison of human cord blood mesenchymal stem cell culture between using human umbilical cord plasma and using fetal bovine serum. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013; 30:1279–1282.14. Pelekanos RA, Sardesai VS, Futrega K, Lott WB, Kuhn M, Doran MR. Isolation and expansion of mesenchymal stem/stromal cells derived from human placenta tissue. J Vis Exp. 2016; (112):DOI: 10.3791/54204. PMID: 27340821. PMCID: 4927767.
Article15. Fei X, Jiang S, Zhang S, Li Y, Ge J, He B, Goldstein S, Ruiz G. Isolation, culture, and identification of amniotic fluid-derived mesenchymal stem cells. Cell Biochem Biophys. 2013; 67:689–694. DOI: 10.1007/s12013-013-9558-z. PMID: 23508888.
Article16. Wouters G, Grossi S, Mesoraca A, Bizzoco D, Mobili L, Cignini P, Giorlandino C. Isolation of amniotic fluid-derived mesenchymal stem cells. J Prenat Med. 2007; 1:39–40. PMID: 22470826. PMCID: 3309337.17. Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, Nguyen TT, Bui AN, Phan NK. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med. 2014; 12:56. DOI: 10.1186/1479-5876-12-56. PMID: 24565047. PMCID: 3939935.
Article18. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article19. Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013; DOI: 10.1155/2013/916136. PMID: 23984420. PMCID: 3741948.
Article20. Iftimia-Mander A, Hourd P, Dainty R, Thomas RJ. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank. 2013; 11:291–298. DOI: 10.1089/bio.2013.0027.
Article21. Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Chang JY, Kim BJ, Jeon HW. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int. 2013; DOI: 10.1155/2013/428726.
Article22. Hendijani F, Sadeghi-Aliabadi H, Haghjooy Javanmard S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton’s jelly matrix. Cell Tissue Bank. 2014; 15:555–565. DOI: 10.1007/s10561-014-9425-1. PMID: 24532125.
Article23. Capelli C, Gotti E, Morigi M, Rota C, Weng L, Dazzi F, Spinelli O, Cazzaniga G, Trezzi R, Gianatti A, Rambaldi A, Golay J, Introna M. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy. 2011; 13:786–801. DOI: 10.3109/14653249.2011.563294. PMID: 21417678.
Article24. Hendijani F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017; 50:DOI: 10.1111/cpr.12334. PMID: 28144997.
Article25. Wolfe M, Tucker A, Reger RL, Prockop DJ. Multipotent stromal cells (hMSCs). Masters JRW, Palsson B, editors. Human Adult Stem Cells. Dordrecht, Netherlands: Springer;2009. p. 45–72. DOI: 10.1007/978-90-481-2269-1_2.
Article26. Tekkatte C, Gunasingh GP, Cherian KM, Sankaranarayanan K. “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int. 2011; DOI: 10.4061/2011/504723.
Article27. Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother. 2013; 40:326–335. DOI: 10.1159/000354061. PMID: 24273486. PMCID: 3822281.
Article28. Díez JM, Bauman E, Gajardo R, Jorquera JI. Culture of human mesenchymal stem cells using a candidate pharmaceutical grade xeno-free cell culture supplement derived from industrial human plasma pools. Stem Cell Res Ther. 2015; 6:28. DOI: 10.1186/s13287-015-0016-2. PMID: 25889980. PMCID: 4396121.
Article29. Tateishi K, Ando W, Higuchi C, Hart DA, Hashimoto J, Nakata K, Yoshikawa H, Nakamura N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical applications. Cell Transplant. 2008; 17:549–557. DOI: 10.3727/096368908785096024. PMID: 18714674.
Article30. Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int. 2007; 31:293–298. DOI: 10.1016/j.cellbi.2006.11.010. PMID: 17208468.
Article31. Salzig D, Leber J, Merkewitz K, Lange MC, Köster N, Czermak P. Attachment, growth, and detachment of human mesenchymal stem cells in a chemically defined medium. Stem Cells Int. 2016; DOI: 10.1155/2016/5246584. PMID: 27006663. PMCID: 4781990.
Article32. Kinzebach S, Bieback K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. Weyand B, Benda C, editors. Mesenchymal stem cells: basics and clinical application. Heidelberg, Berlin: Springer;2013. p. 33–57.
Article33. Song HJ, Zhang P, Guo XJ, Liao LM, Zhou ZM, Sha JH, Cui YG, Ji H, Liu JY. The proteomic analysis of human neonatal umbilical cord serum by mass spectrometry. Acta Pharmacol Sin. 2009; 30:1550–1558. DOI: 10.1038/aps.2009.140. PMID: 19890362. PMCID: 4003003.
Article34. Schnitzler AC, Verma A, Kehoe DE, Jing D, Murrell JR, Der KA, Aysola M, Rapiejko PJ. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochemical Engineering Journal. 2016; 108:3–13. DOI: 10.1016/j.bej.2015.08.014.
Article35. Phadnis SM, Joglekar MV, Venkateshan V, Ghaskadbi SM, Hardikar AA, Bhonde RR. Human umbilical cord blood serum promotes growth, proliferation, as well as differentiation of human bone marrow-derived progenitor cells. In Vitro Cell Dev Biol Anim. 2006; 42:283–286.
Article36. Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014; 16:170–180. DOI: 10.1016/j.jcyt.2013.11.004. PMID: 24438898.
Article37. Van Pham P, Truong NC, Le PT, Tran TD, Vu NB, Bui KH, Phan NK. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016; 17:289–302. DOI: 10.1007/s10561-015-9541-6.
Article38. Shetty P, Cooper K, Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci. 2010; 4:14–24. DOI: 10.4103/0973-6247.59386. PMID: 20376261. PMCID: 2847339.
Article39. Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy. 2009; 11:874–885. DOI: 10.3109/14653240903188947. PMID: 19903100.
Article40. Tekkatte C, Vidyasekar P, Kapadia NK, Verma RS. Enhancement of adipogenic and osteogenic differentiation of human bone-marrow-derived mesenchymal stem cells by supplementation with umbilical cord blood serum. Cell Tissue Res. 2012; 347:383–395. DOI: 10.1007/s00441-012-1328-5. PMID: 22311206.
Article41. Cooper K, SenMajumdar A, Viswanathan C. Derivation, expansion and characterization of clinical grade mesenchymal stem cells from umbilical cord matrix using cord blood serum. Int J Stem Cells. 2010; 3:119–128. DOI: 10.15283/ijsc.2010.3.2.119. PMID: 24855549. PMCID: 4021805.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Minimal Cube Explant Provides Optimal Isolation Condition of Mesenchymal Stem Cells from Umbilical Cord
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood