Nutr Res Pract.
2014 Oct;8(5):501-508. 10.4162/nrp.2014.8.5.501.
Anti-inflammatory effects of Rubus coreanus Miquel through inhibition of NF-kappaB and MAP Kinase
- Affiliations
-
- 1Department of Home Economics Education, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul 156-756, Korea. jjhkim@cau.ac.kr
- 2Functional Food & Nutrition Division, Rural Development Administration, Gyeonggi 441-853, Korea.
- 3Department of Food and Nutrition, College of Human Ecology, Yonsei Universiy, Seoul 120-749, Korea.
- 4Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 120-750, Korea.
- 5Department of Food Science & Technology, Chung-Ang University, Gyeonggi 456-756, Korea.
- 6College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- 7Department of Life Science, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul 156-756, Korea.
- KMID: 2313770
- DOI: http://doi.org/10.4162/nrp.2014.8.5.501
Abstract
- BACKGROUND/OBJECTIVES
Rubus Coreanus Miquel (RCM), used as a traditional Korean medicine, reduces chronic inflammatory diseases such as cancer and rheumatoid arthritis. However, its mechanism has not been elucidated. In this study, we examine the anti-inflammatory effects of RCM and their possible mechanisms using RAW 264.7 cells.
MATERIALS/METHODS
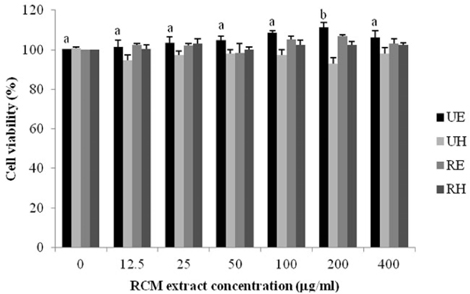
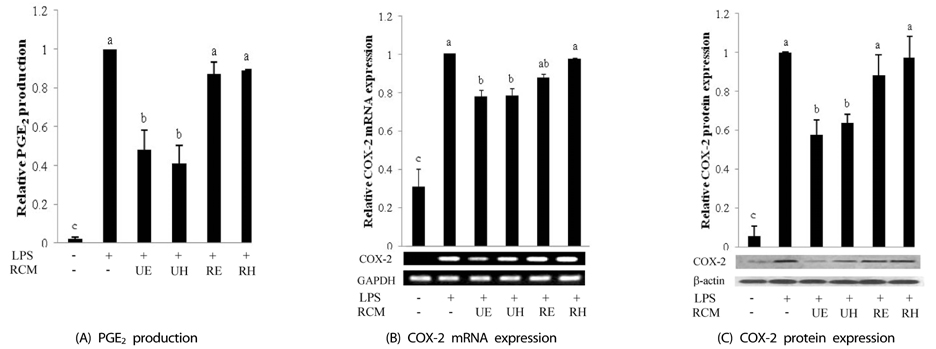
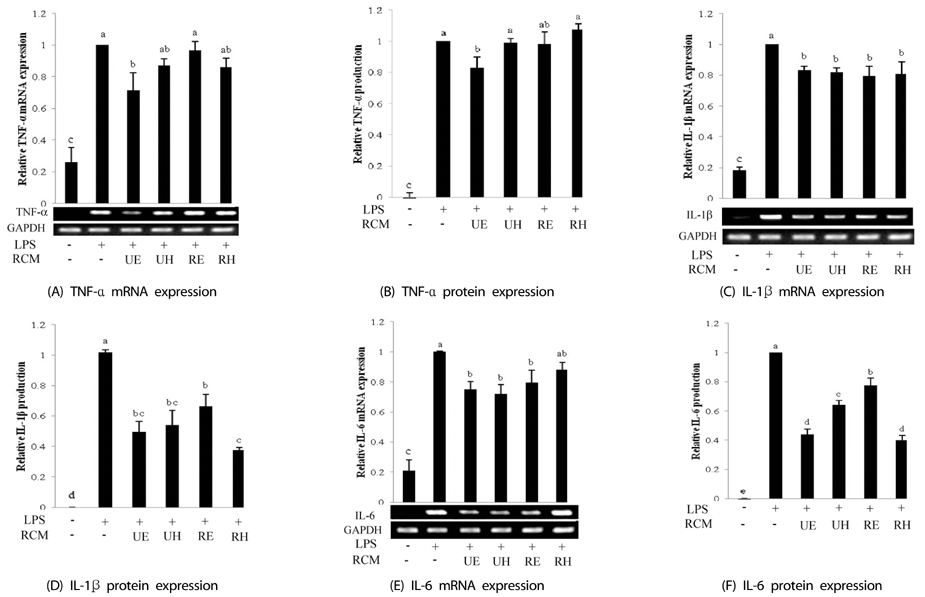
Unripe RCM ethanol extract (UE), unripe RCM water extract (UH), ripe RCM ethanol extract (RE), and ripe RCM water extract (RH) were prepared. Inflammatory response was induced with LPS treatment, and expression of pro-inflammatory mediators (iNOS, COX-2, TNF-alpha, IL-1beta, and IL-6) and NO and PGE2 productions were assessed. To determine the anti-inflammatory mechanism of RCM, we measured NF-kappaB and MAPK activities.
RESULTS
UE and UH treatment significantly reduced NF-kappaB activation and JNK and p38 phosphorylation and reduced transcriptional activities decreased iNOS, COX-2, and pro-inflammatory cytokines expressions, and NO and PGE2 productions. RE and RH treatments reduced IL-1beta and IL-6 expressions through suppressions of JNK and p38 phosphorylation.
CONCLUSIONS
In this study, we showed that RCM had anti-inflammatory effects by suppression of pro-inflammatory mediator expressions. Especially, unripe RCM showed strong anti-inflammatory effects through suppression of NF-kappaB and MAPK activation. These findings suggest that unripe RCM might be used as a potential functional material to reduce chronic inflammatory responses.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Laroux FS. Mechanisms of inflammation: the good, the bad and the ugly. Front Biosci. 2004; 9:3156–3162.
Article2. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997; 336:1066–1071.
Article3. Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997; 17:3–9.
Article4. Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011; 12:715–723.
Article5. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353:1649–1652.
Article6. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Centers for Disease Control and Prevention. American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107:499–511.7. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.8. Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998; 38:981–993.
Article9. Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995; 15:2809–2818.
Article10. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994; 10:405–455.11. Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999; 97:124–129.
Article12. Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13:85–94.
Article13. Kim EJ, Lee YJ, Shin HK, Park JH. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition. 2005; 21:1141–1148.
Article14. Ra JC, Lee HY, Choi MK, Park HG, Kang KS. Effect of decreasing body weight with plant extracts containing Rubi fructus. J Toxicol Public Health. 2004; 20:167–172.15. Yang HM, Oh SM, Lim SS, Shin HK, Oh YS, Kim JK. Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother Res. 2008; 22:102–107.
Article16. Yang HM, Lim SS, Lee YS, Shin HK, Oh YS, Kim JK. Comparison of the anti-inflammatory effects of the extracts from Rubus coreanus and Rubus occidentalis. Korean J Food Sci Technol. 2007; 39:342–347.17. Kim Y, Kim J, Lee SM, Lee HA, Park S, Kim Y, Kim JH. Chemopreventive effects of Rubus coreanus Miquel on prostate cancer. Biosci Biotechnol Biochem. 2012; 76:737–744.18. Kim YH, Choi JH, Rim HK, Kang HJ, Chang SG, Park JH, Park HJ, Choi JW, Kim SD, Lee KT. 23-Hydroxytormentic acid and nigaichgoside f(1) isolated from Rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK(1) cells. Biol Pharm Bull. 2011; 34:906–911.
Article19. Lee J, Dossett M, Finn CE. Rubus fruit phenolic research: the good, the bad, and the confusing. Food Chem. 2012; 130:785–796.
Article20. Pang KC, Kim MS, Lee MW. Hydrolyzable tannins from the fruits of Rubus coreanum. Korean J Pharmacogn. 1996; 27:366–370.21. Kim HS, Park SJ, Hyun SH, Yang SO, Lee J, Auh JH, Kim JH, Cho SM, Marriott PJ, Choi HK. Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1H-NMR using multiple solvent systems. Food Res Int. 2011; 44:1977–1988.22. Ellis CL, Edirisinghe I, Kappagoda T, Burton-Freeman B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J Atheroscler Thromb. 2011; 18:318–327.
Article23. Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, Blay M. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009; 20:210–218.
Article24. Seymour EM, Lewis SK, Urcuyo-Llanes DE, Tanone II, Kirakosyan A, Kaufman PB, Bolling SF. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009; 12:935–942.
Article25. Kim HH, Choi PH, Yoo JS, Jeon H, Chae BS, Park JS, Kim SH, Shin TY. Ripe fruit of Rubus coreanus inhibits mast cell-mediated allergic inflammation. Int J Mol Med. 2012; 29:303–310.
Article26. Sangiovanni E, Vrhovsek U, Rossoni G, Colombo E, Brunelli C, Brembati L, Trivulzio S, Gasperotti M, Mattivi F, Bosisio E, Dell'Agli M. Ellagitannins from Rubus berries for the control of gastric inflammation: in vitro and in vivo studies. PLoS One. 2013; 8:e71762.
Article27. Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006; 281:30678–30683.
Article28. Kim JM, Shin M. Characteristics of Rubus coreanus Miq. Fruits at different ripening stages. Korean J Food Sci Technol. 2011; 43:341–347.
Article29. Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000; 48:140–146.
Article30. González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011; 51:331–362.
Article31. Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999; 5:439–447.
Article32. Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004; 24:7806–7819.
Article33. Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl). 1996; 74:589–607.
Article34. Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:471–479.
Article35. Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993; 54:171–178.
Article36. Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004; 500:255–266.
Article37. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997; 15:323–350.
Article38. Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007; 121:2357–2363.
Article39. Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998; 12:1063–1073.
Article40. Lin CC, Lee IT, Yang YL, Lee CW, Kou YR, Yang CM. Induction of COX-2/PGE(2)/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: role of TLR4-dependent NADPH oxidase activation. Free Radic Biol Med. 2010; 48:240–254.
Article41. Willoughby DA, Moore AR, Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet. 2000; 355:646–648.
Article42. Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY, Zhang SL, Huang ZJ, Xiong FL, Li CM. Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J Ethnopharmacol. 2009; 123:97–105.
Article43. Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997; 2:d12–d26.
Article44. Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000; 119:1209–1218.
Article45. Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002; 96:23–43.
Article46. Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010; 24:157–165.
Article47. Schubert SY, Neeman I, Resnick N. A novel mechanism for the inhibition of NF-κB activation in vascular endothelial cells by natural antioxidants. FASEB J. 2002; 16:1931–1933.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment for Two Cases of Acne Vulgaris with Rubus Coreanus Miquel Extract
- Growth inhibition effect of Rubus coreanus Miquel on Candida albicans
- A Study of Potential Application of Rubus coreanus Miquel Extract for Seborrheic Dermatitis Treatment
- Emodin Isolated from Polygoni cuspidati Radix Inhibits TNF-alpha and IL-6 Release by Blockading NF-kappaB and MAP Kinase Pathways in Mast Cells Stimulated with PMA Plus A23187
- ASK1 is Involved in EBV LMP1-induced NF-kappaB Activation