Nutr Res Pract.
2014 Apr;8(2):172-176.
Cordyceps militaris alleviates non-alcoholic fatty liver disease in ob/ob mice
- Affiliations
-
- 1Department of Smart Food and Drugs, School of Food and Life Science, Inje University, 197 Inje-ro, Gimhae, Gyeongnam 621-749, Korea. fdsnkiji@inje.ac.kr
- 2Laboratory of Nutritional Analysis, Hurom Co., Ltd., Gyeongnam 660-701, Korea.
- 3Department of Biotechnology, Dong-A University, Busan 604-714, Korea.
- 4Medi-Farm Industrialization Research Center, Dong-A University, Busan 604-714, Korea.
Abstract
- BACKGROUND/OBJECTIVES
Non-alcoholic fatty liver disease (NAFLD) is becoming an important public health problem as metabolic syndrome and type 2 diabetes have become epidemic. In this study we investigated the protective effect of Cordyceps militaris (C. militaris) against NAFLD in an obese mouse model.
MATERIALS/METHODS
Four-week-old male ob/ob mice were fed an AIN-93G diet or a diet containing 1% C. militaris water extract for 10 weeks after 1 week of adaptation. Serum glucose, insulin, free fatty acid (FFA), alanine transaminase (ALT), and proinflammatory cytokines were measured. Hepatic levels of lipids, glutathione (GSH), and lipid peroxide were determined.
RESULTS
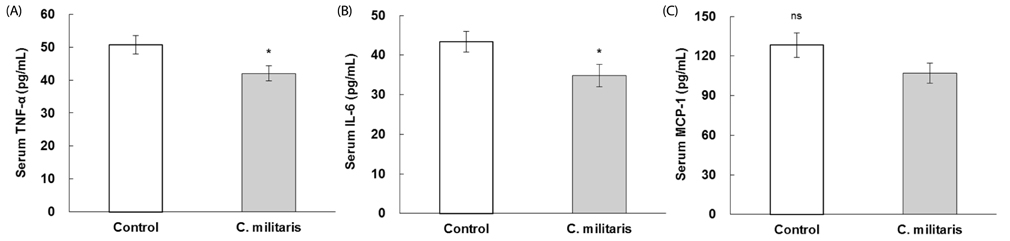
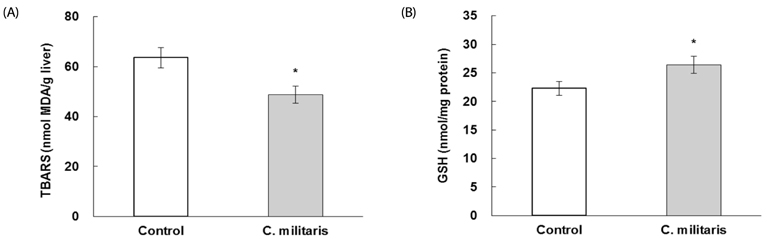
Consumption of C. militaris significantly decreased serum glucose, as well as homeostasis model assessment for insulin resistance (HOMA-IR), in ob/ob mice. In addition to lowering serum FFA levels, C. militaris also significantly decreased hepatic total lipids and triglyceride contents. Serum ALT activities and tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) levels were reduced by C. militaris. Consumption of C. militaris increased hepatic GSH and reduced lipid peroxide levels.
CONCLUSIONS
These results indicate that C. militaris can exert protective effects against development of NAFLD, partly by reducing inflammatory cytokines and improving hepatic antioxidant status in ob/ob mice.
Keyword
MeSH Terms
-
Alanine Transaminase
Animals
Blood Glucose
Cordyceps*
Cytokines
Diet
Fatty Liver*
Glutathione
Homeostasis
Humans
Insulin
Insulin Resistance
Interleukin-6
Male
Mice*
Mice, Obese
Public Health
Triglycerides
Tumor Necrosis Factor-alpha
Water
Alanine Transaminase
Cytokines
Glutathione
Insulin
Interleukin-6
Tumor Necrosis Factor-alpha
Water
Figure
Reference
-
1. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:1705–1725.
Article2. Petta S, Muratore C, Craxì A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis. 2009; 41:615–625.
Article3. Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998; 114:842–845.
Article4. Salt WB 2nd. Nonalcoholic fatty liver disease (NAFLD): a comprehensive review. J Insur Med. 2004; 36:27–41.5. Ahmed MH, Abu EO, Byrne CD. Non-Alcoholic Fatty Liver Disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes. 2010; 4:129–137.
Article6. Day CP. From fat to inflammation. Gastroenterology. 2006; 130:207–210.
Article7. Marchesini G, Forlani G. NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology. 2002; 35:497–499.
Article8. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008; 19:371–379.
Article9. Lebovics E, Rubin J. Non-alcoholic fatty liver disease (NAFLD): why you should care, when you should worry, what you should do. Diabetes Metab Res Rev. 2011; 27:419–424.
Article10. Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007; 65:S57–S63.
Article11. Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013; 92:114–118.
Article12. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
Article13. Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008; 69:1469–1495.
Article14. Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005; 57:1509–1519.
Article15. Yu HM, Wang BS, Huang SC, Duh PD. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J Agric Food Chem. 2006; 54:3132–3138.
Article16. Zhan Y, Dong CH, Yao YJ. Antioxidant activities of aqueous extract from cultivated fruit-bodies of Cordyceps militaris (L.) Link in vitro. J Integr Plant Biol. 2006; 48:1365–1370.
Article17. Wang BS, Lee CP, Chen ZT, Yu HM, Duh PD. Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. J Funct Foods. 2012; 4:489–495.
Article18. Jo WS, Choi YJ, Kim HJ, Lee JY, Nam BH, Lee JD, Lee SW, Seo SY, Jeong MH. The anti-inflammatory effects of water extract from Cordyceps militaris in murine macrophage. Mycobiology. 2010; 38:46–51.
Article19. Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006; 87:1–16.
Article20. Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008; 149:3549–3558.
Article21. Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008; 138:323–331.
Article22. Massart J, Robin MA, Noury F, Fautrel A, Lettéron P, Bado A, Eliat PA, Fromenty B. Pentoxifylline aggravates fatty liver in obese and diabetic ob/ob mice by increasing intestinal glucose absorption and activating hepatic lipogenesis. Br J Pharmacol. 2012; 165:1361–1374.
Article23. Zhang G, Huang Y, Bian Y, Wong JH, Ng TB, Wang H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol. 2006; 72:1152–1156.
Article24. Choi HN, Kang MJ, Jeong SM, Seo MJ, Kang BW, Jeong YK, Kim JI. Effect of Dongchunghacho (Cordyceps militaris) on hyperglycemia and dyslipidemia in type 2 diabetic db/db mice. Food Sci Biotechnol. 2012; 21:1157–1162.
Article25. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article26. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
Article27. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article28. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article29. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.
Article30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
Article31. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995; 269:543–546.
Article32. Koteish A, Mae Diehl A. Animal models of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002; 16:679–690.
Article33. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008; 294:E15–E26.
Article34. Choi SB, Park CH, Choi MK, Jun DW, Park S. Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotechnol Biochem. 2004; 68:2257–2264.
Article35. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989; 83:1168–1173.
Article36. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005; 115:1343–1351.
Article37. Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol (1985). 2003; 94:2127–2134.
Article38. Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994; 43:1271–1278.
Article39. Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003; 278:13740–13746.
Article40. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006; 116:1494–1505.
Article41. Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000; 378:259–268.
Article42. Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3'-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012; 47:979–987.
Article43. Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999; 274:33627–33636.
Article44. Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003; 57:145–155.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Ingredients of Cordyceps militaris
- Exendin-4 Improves Nonalcoholic Fatty Liver Disease by Regulating Glucose Transporter 4 Expression in ob/ob Mice
- Increased Plasma Dipeptidyl Peptidase IV Activities in ob/ob Mice
- Hog millet (Panicum miliaceum L.)-supplemented diet ameliorates hyperlipidemia and hepatic lipid accumulation in C57BL/6J-ob/ob mice
- Effect of sweet pumpkin powder on lipid metabolism in leptin-deficient mice