Nutr Res Pract.
2011 Dec;5(6):511-519.
Hog millet (Panicum miliaceum L.)-supplemented diet ameliorates hyperlipidemia and hepatic lipid accumulation in C57BL/6J-ob/ob mice
- Affiliations
-
- 1Functional Food & Nutrition Division, National Academy of Agricultural Science, Rural Develpoment Administration, Seodun-dong, Gwonseon-gu, Suwon-si 88-2, Gyeonggi 441-707, Korea. dpark@korea.kr
Abstract
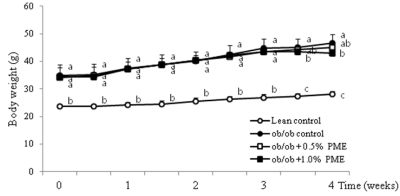
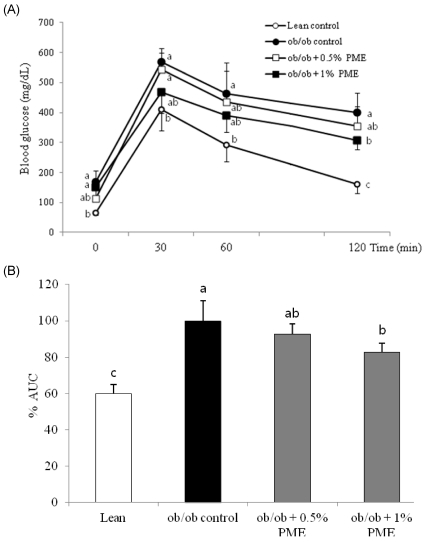
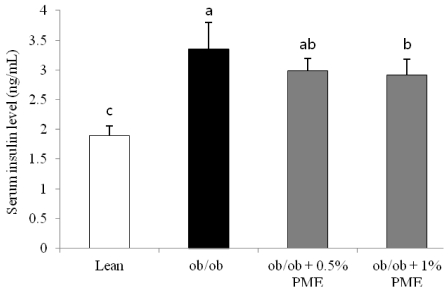
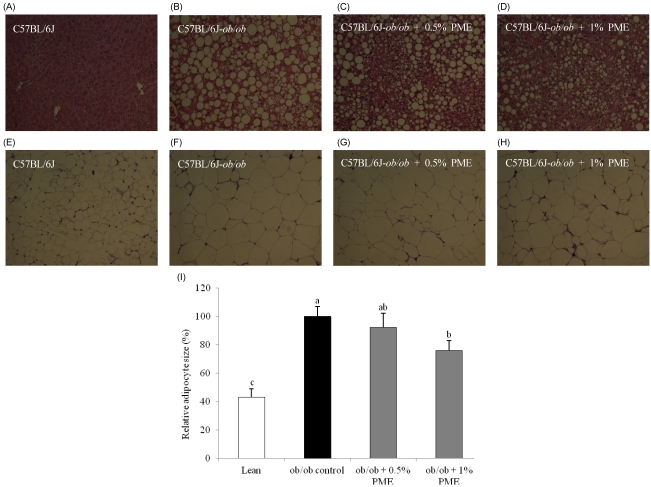
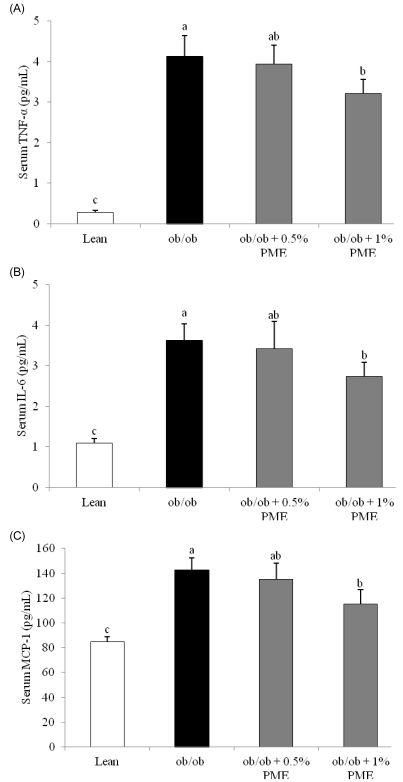
- Dietary intake of whole grains reduces the incidence of chronic diseases such as obesity, diabetes, cardiovascular disease, and cancer. In an earlier study, we showed that Panicum miliaceum L. extract (PME) exhibited the highest anti-lipogenic activity in 3T3-L1 cells among extracts of nine different cereal grains tested. In this study, we hypothesized that PME in the diet would lead to weight loss and augmentation of hyperlipidemia by regulating fatty acid metabolism. PME was fed to ob/ob mice at 0%, 0.5%, or 1% (w/w) for 4 weeks. After the experimental period, body weight changes, blood serum and lipid profiles, hepatic fatty acid metabolism-related gene expression, and white adipose tissue (WAT) fatty acid composition were determined. We found that the 1% PME diet, but not the 0.5%, effectively decreased body weight, liver weight, and blood triglyceride and total cholesterol levels (P < 0.05) compared to obese ob/ob mice on a normal diet. Hepatic lipogenic-related gene (PPARalpha, L-FABP, FAS, and SCD1) expression decreased, whereas lipolysis-related gene (CPT1) expression increased in animals fed the 1% PME diet (P < 0.05). Long chain fatty acid content and the ratio of C18:1/C18:0 fatty acids decreased significantly in adipose tissue of animals fed the 1% PME diet (P < 0.05). Serum inflammatory mediators also decreased significantly in animals fed the 1% PME diet compared to those of the ob/ob control group (P < 0.05). These results suggest that PME is useful in the chemoprevention or treatment of obesity and obesity-related disorders.
MeSH Terms
Figure
Reference
-
1. Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010; 45:199–214. PMID: 20218765.2. Kantartzis K, Schick F, Häring HU, Stefan N. Environmental and genetic determinants of fatty liver in humans. Dig Dis. 2010; 28:169–178. PMID: 20460907.
Article3. Ntambi JM, Miyazaki M, Dobrzyn A. Regulation of stearoyl-CoA desaturase expression. Lipids. 2004; 39:1061–1065. PMID: 15726820.
Article4. Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005; 2:251–261. PMID: 16213227.
Article5. Sjögren P, Sierra-Johnson J, Gertow K, Rosell M, Vessby B, de Faire U, Hamsten A, Hellenius ML, Fisher RM. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia. 2008; 51:328–335. PMID: 18030445.
Article6. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010; 23:65–134. PMID: 20565994.
Article7. Park MY, Seo DW, Lee JY, Sung MK, Lee YM, Jang HH, Choi HY, Kim JH, Park DS. Effects of Panicum miliaceum L. extract on adipogenic transcription factors and fatty acid accumulation in 3T3-L1 adipocytes. Nutr Res Pract. 2011; 5:192–197. PMID: 21779521.8. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951. PMID: 8229312.
Article9. Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009; 52:882–890. PMID: 19252892.10. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.11. Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, Vajreswari A. Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab (Lond). 2009; 6:27. PMID: 19519902.
Article12. Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999; 274:5692–5700. PMID: 10026188.
Article13. Lindström P. beta-cell function in obese-hyperglycemic mice [ob/ob Mice]. Adv Exp Med Biol. 2010; 654:463–477. PMID: 20217510.14. von Eynatten M, Baumann M, Heemann U, Zdunek D, Hess G, Nawroth PP, Bierhaus A, Humpert PM. Urinary L-FABP and anaemia: distinct roles of urinary markers in type 2 diabetes. Eur J Clin Invest. 2010; 40:95–102. PMID: 19912308.
Article15. Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair MG, Peters JM, Busik JV, Olson LK, Jump DB. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006; 47:2028–2041. PMID: 16790840.
Article16. Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999; 40:1549–1558. PMID: 10484602.
Article17. López-Parra M, Titos E, Horrillo R, Ferré N, González-Périz A, Martínez-Clemente M, Planagumà A, Masferrer J, Arroyo V, Clària J. Regulatory effects of arachidonate 5-lipoxygenase on hepatic microsomal TG transfer protein activity and VLDL-triglyceride and apoB secretion in obese mice. J Lipid Res. 2008; 49:2513–2523. PMID: 18645210.
Article18. Fernandez-Real JM, Menendez JA, Moreno-Navarrete JM, Blüher M, Vazquez-Martin A, Vázquez MJ, Ortega F, Diéguez C, Frühbeck G, Ricart W, Vidal-Puig A. Extracellular fatty acid synthase: a possible surrogate biomarker of insulin resistance. Diabetes. 2010; 59:1506–1511. PMID: 20299470.
Article19. McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997; 244:1–14. PMID: 9063439.20. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000; 279:E1039–E1044. PMID: 11052958.
Article21. Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007; 581:431–444. PMID: 17331998.
Article22. Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. 1997; 337:396–407. PMID: 9241130.
Article23. Wasserman DH, Cherrington AD. Hepatic fuel metabolism during muscular work: role and regulation. Am J Physiol. 1991; 260:E811–E824. PMID: 2058658.
Article24. Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997; 94:2557–2562. PMID: 9122234.
Article25. Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010; 11:145–156. PMID: 20173393.
Article26. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010; 17:332–341. PMID: 20124732.
Article27. Park MY, Kim JH, Park DS. Anti-inflammatory activities of hog millet (Panicum miliaceum L.) in murine macrophages through IRAK-4 signaling. Korean J Food Nutr. 2011; 24:268–272.28. Rekhter M, Staschke K, Estridge T, Rutherford P, Jackson N, Gifford-Moore D, Foxworthy P, Reidy C, Huang XD, Kalbfleisch M, Hui K, Kuo MS, Gilmour R, Vlahos CJ. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008; 367:642–648. PMID: 18190779.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cyclo-His-Pro-enriched yeast hydrolysate on blood glucose levels and lipid metabolism in obese diabetic ob/ob mice
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Effect of sweet pumpkin powder on lipid metabolism in leptin-deficient mice
- Increased Plasma Dipeptidyl Peptidase IV Activities in ob/ob Mice
- Anti-obesity activity of diglyceride containing conjugated linoleic acid in C57BL/6J ob/ob mice