J Breast Cancer.
2008 Dec;11(4):161-171. 10.4048/jbc.2008.11.4.161.
The Expression of Heat Shock Protein 60 kDa in Tissues and Cell Lines of Breast Cancer
- Affiliations
-
- 1Department of Surgery, Gachon University of Medicine and Science, Incheon, Korea. hgjh@gilhospital.com
- 2Department of Molecular Biology, Gachon University of Medicine and Science, Incheon, Korea.
- KMID: 2286581
- DOI: http://doi.org/10.4048/jbc.2008.11.4.161
Abstract
-
PURPOSE: Breast cancer has been reported as the most common cancer of women in the United States, Western Europe and Korea and about 210,000 and 10,000 women in United States and Korea every year, respectively are diagnosed with it. Breast cancer is curable with an early diagnosis, and many researchers have made efforts to find a marker for this malady, heat shock protein (HSP) consists of 6 groups, it is highly preserved throughout both the prokaryotic and eukaryotic cells and it acts as a molecular chaperone that's involved in protein folding. HSPs have been recently reported to be related with breast cancer. In this study, we investigated the changes of expression of HSP60 in tissues and cell lines of breast cancer.
METHODS
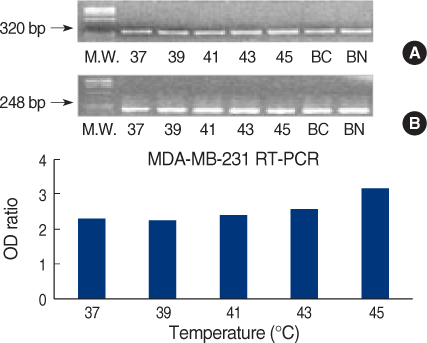
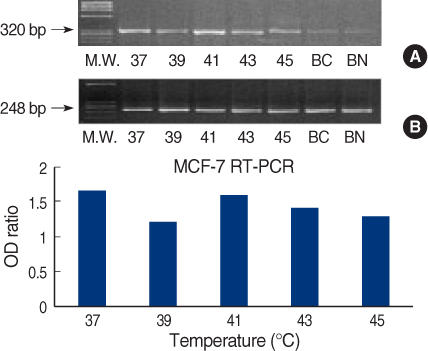
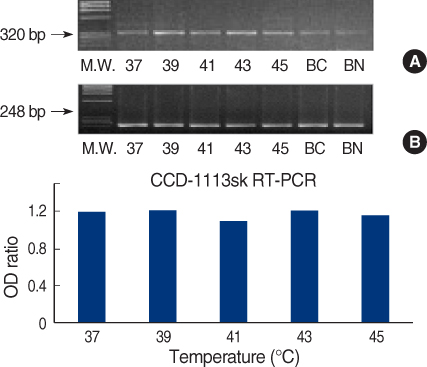
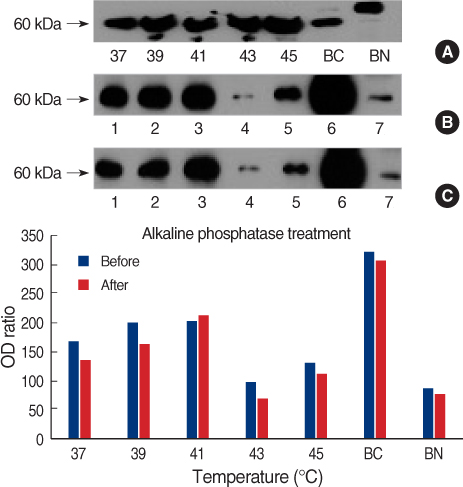
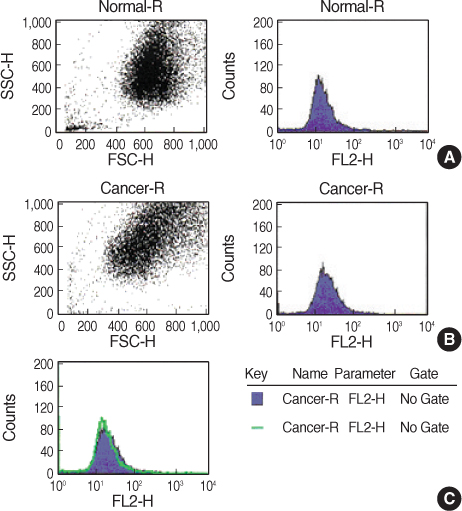
We obtained breast cancer tissues and normal tissues from breast cancer patients, and we purchased several cancer cell lines from American tissue culture correction. We treated the tissues and the cell lines of human breast cancer with heat shock protein. Proteins and mRNAs were isolated from the tissues and the cells and then we performed Western blotting, reverse transcriptase-Polymerase chain Reaction and fluorescence activated cell sorter analysis on them.
RESULTS
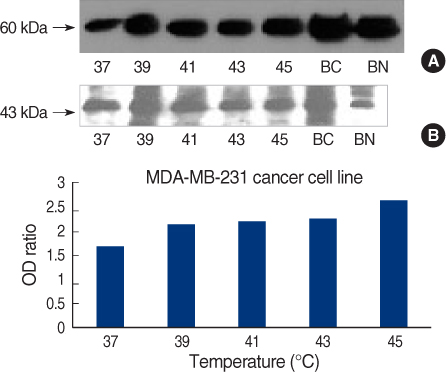
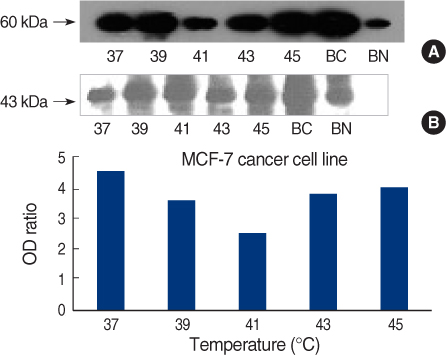
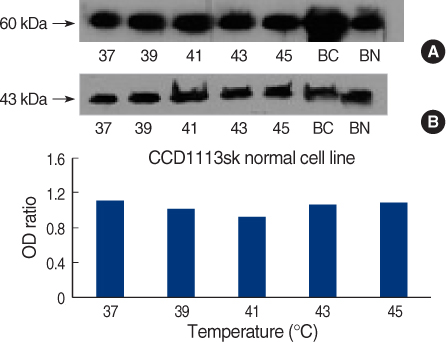
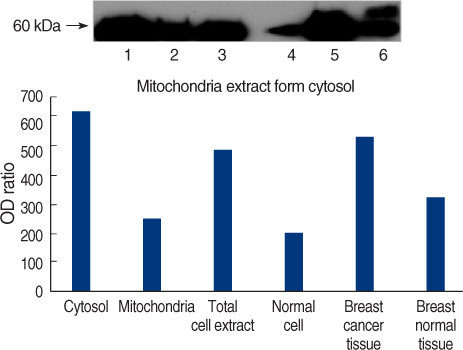
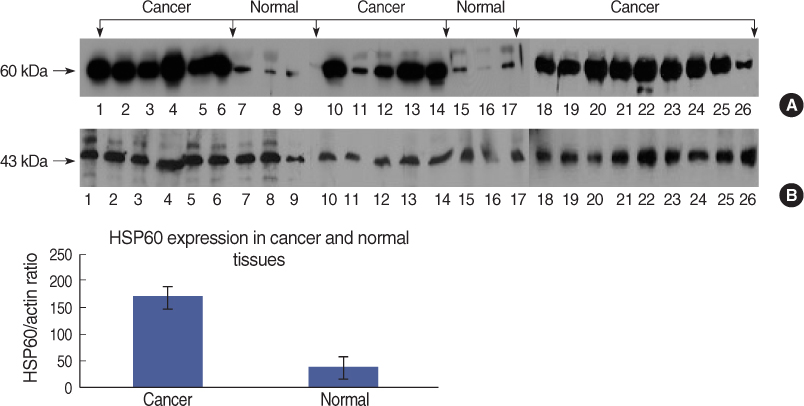
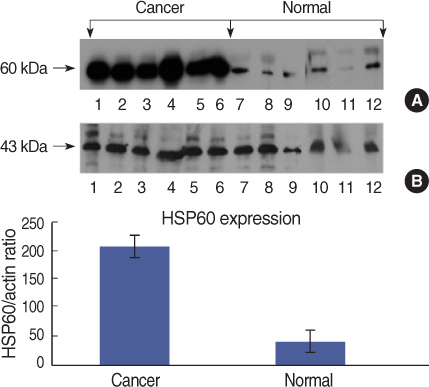
On Western blot, HSP60 was more overexpressed in the tissues and the cell lines of breast cancer than in the normal breast tissues and cell lines. The expression of HSP60 showed 2 types of molecular weight differences in the tissues and cell lines of breast cancer, and specifically, low HSP60 was over-expressed in the cancer tissues. There was no difference between the breast cancer cell lines and the normal cell lines in the expressions of HSP60 mRNA, according to the treatment with heat shock. Also, there was no relationship between phosphorylation and the structural difference of HSP60 protein, according to HSP60 protein's molecular weight. The expression of HSP60 has been mostly reported at the mitochondria; however, in this study, it was more predominantly detected at the cytoplasm than at the mitochondria in the breast cancer cell lines.
CONCLUSION
We conclude that HSP60 may be used as a diagnostic marker for breast cancer. Detailed investigation of the usefulness and significance of the HSP60 expression as a prognostic factor is required in further studies.
Keyword
MeSH Terms
-
Blotting, Western
Breast
Breast Neoplasms
Cell Line
Chaperonin 60
Cytoplasm
Early Diagnosis
Eukaryotic Cells
Europe
Female
Fibrinogen
Fluorescence
Heat-Shock Proteins
Hot Temperature
Humans
Korea
Mitochondria
Molecular Chaperones
Molecular Weight
Phosphorylation
Protein Folding
Proteins
RNA, Messenger
Shock
United States
Chaperonin 60
Fibrinogen
Heat-Shock Proteins
Molecular Chaperones
Proteins
RNA, Messenger
Figure
Reference
-
1. Yun HS. Nationwide Korean Breast Cancer Data of 2002. J Korean Breast Cancer Soc. 2004. 7:72–83.
Article2. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtiqian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994. 266:66–71.
Article3. Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002. 52:23–24.
Article4. Ritossa FA. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962. 18:571–573.
Article5. Tissiere A, Mitchell HK, Tracy U. Protein synthesis in salivary glands of Dorsophila melanogaster: relation to chromosomal puffs. J Mol Biol. 1974. 84:389–398.6. Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998. 92:351–366.
Article7. Cappello F, Bellafiore M, Palma A, Marciano V. Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology. 2002. 70:83–88.
Article8. Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999. 31:261–271.
Article9. Morano KA, Thiele DJ. Heat shock function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 1999. 7:271–282.10. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000. 92:1564–1572.
Article11. Knowlton AA. The role of heat shock proteins in the heart. J Mol Cell Cardiol. 1995. 27:121–131.
Article12. Schneider J, Jimenez E, Marenbach K, Romero H, Mark D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer, correlation with survival in a series of 247 patients. Anticancer Res. 1999. 19:2141–2146.13. Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991. 167:109–123.
Article14. Bradford MM. A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binging. Anal Biochem. 1976. 72:248–254.
Article15. Itoh H, Kobayashi R, Wakui H, Komatsuda A, Ohtani H, Miura AB, et al. Mammalian 60-kDa stress protein (chaperonin homolog). identification, biochemical properties, and localization. J Biol Chem. 1995. 270:13429–13435.16. Franzen B, Linder S, Alaiya AA, Eriksson E, Fujioka K, Berman AC, et al. Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis. 1997. 18:582–587.
Article17. Morimoto RI. Heat shock: the role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cells. 1991. 3:295–301.18. Itoh H, Komatsuda A, Ohtani H, Wakui H, Imai H, Sawada K, et al. Mammalian HSP60 is quickly sorted into the mitochondria under conditions of dehydration. Eur J Biochem. 2002. 269:5931–5938.
Article19. Cicconi R, Delpino A, Piselli P, Castelli M, Vismara D. Expression of 60 kDa heat shock protein (HSP60) on plasma membrane of Daudi cells. Mol Cell Biochem. 2004. 259:1–7.
Article20. Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999. 248:30–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Heat Shock Proteins 70/90 as Potential Molecular Therapeutic Targets in Breast Cancer
- The Expression of a Novel 90 kDa Stress Protein in Human Malignant Neoplasms
- The Role of Inducible 70-kDa Heat Shock Protein in Cell Cycle Control, Differentiation, and Apoptotic Cell Death of the Human Myeloid Leukemic HL-60 Cells
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis