J Breast Cancer.
2007 Dec;10(4):231-240. 10.4048/jbc.2007.10.4.231.
The Role of Heat Shock Proteins 70/90 as Potential Molecular Therapeutic Targets in Breast Cancer
- Affiliations
-
- 1Department of Surgery, Hallym University College of Medicine, Anyang, Korea. lskim0503@hallym.ac.kr
- KMID: 2286590
- DOI: http://doi.org/10.4048/jbc.2007.10.4.231
Abstract
-
PURPOSE: Heat shock proteins (hsps) are molecular chaperones that are synthesized by cells in response to various stress conditions. The expression of hsps have been shown to be associated with carcinogenesis and the expression of hsps have been implicated in the biological behavior of tumors. Recently, hsps have emerged as novel molecular targets in anticancer protocols. The objectives of this study were to investigate the significance of hsp 70/90 in breast carcinogenesis and effect of geldanamycin (a blocker of hsp 90) and quercetin (a blocker of hsp 70) on growth inhibition in different breast cancer cell lines.
METHODS
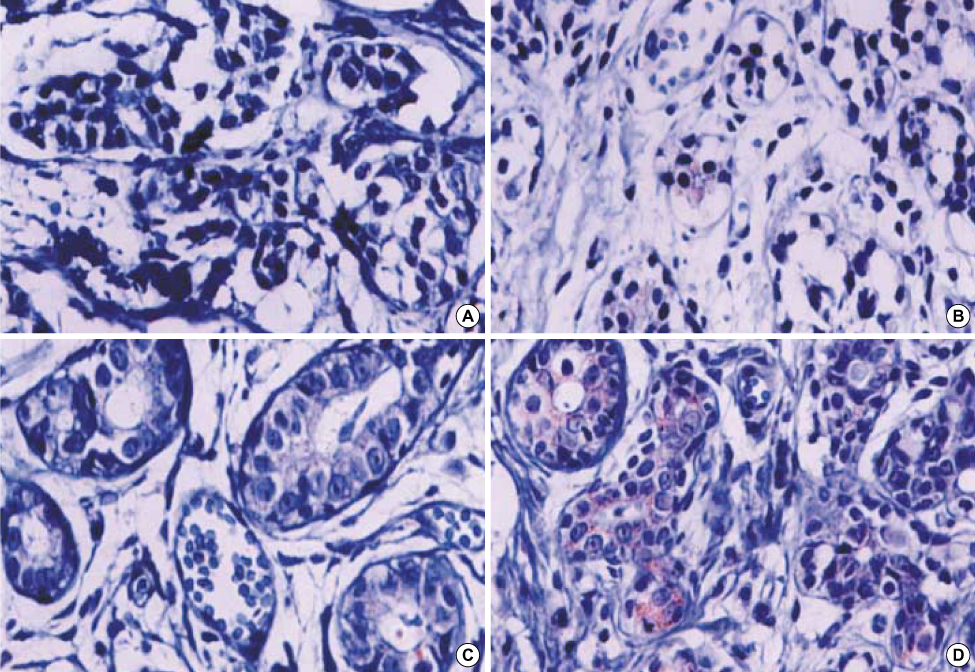
Breast tissues from 82 patients were obtained between June 2003 and May 2005 at the Department of Surgery, Hallym University Hospital. Expression of hsp 70/90 was studied by immunohistochemistry (IHC) on tissue sections from 63 breast carcinomas and 19 benign breast tissues. Both cytoplasmic and nuclear expression was measured. Expression of hsp 70/90 was also analyzed by use of a Western blot with the breast cancer cell lines. We next investigated the effects of blockers of hsp 70/90 on cell growth of the human breast cancer cell lines.
RESULTS
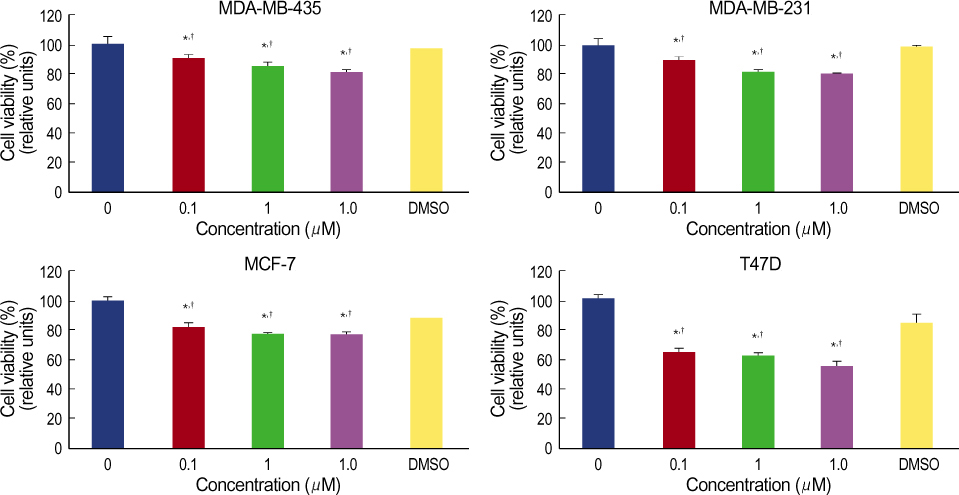
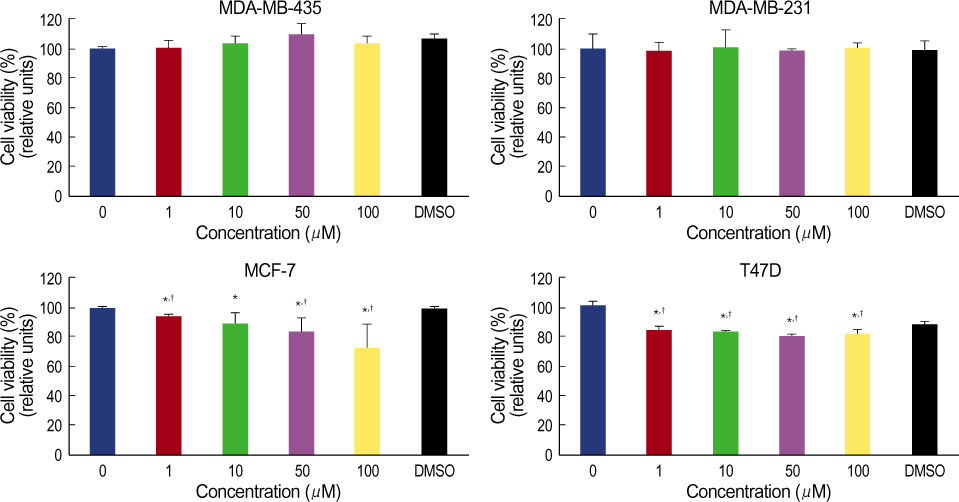
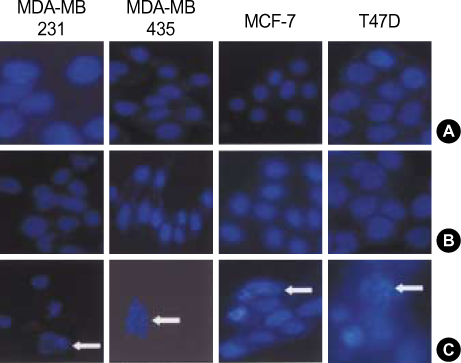
More prominent hsp 90 expression was observed in malignant tissue than in benign tissue by both cytoplasmic and nuclear IHC staining (p<0.001, p<0.001). Nuclear hsp 90 expression was associated with a positive lymph node status (p=0.003) and the presence of poorly differentiated tumors (p=0.028). Expression of hsp 70 was not different in malignant and benign tissues as determined by both cytoplasmic and nuclear IHC staining. The breast cancer cell lines all expressed hsp 70/90. Geldanamycin markedly inhibited the cell growth of these breast cancer cell lines in a dosedependent manner and induced apoptosis in the cell lines. Quercetin inhibited cell growth of the cell lines less efficiently.
CONCLUSION
The expression of hsp 90 was associated with breast carcinogenesis and the presence of more aggressive tumors. Geldanamycin inhibited cell growth of hsp 90 expressing breast cancer cell lines. We suggest that Hsp 90 may be a possible molecular target against breast cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Heat Shock Protein as Molecular Targets for Breast Cancer Therapeutics
Lee Su Kim, Jun Ho Kim
J Breast Cancer. 2011;14(3):167-174. doi: 10.4048/jbc.2011.14.3.167.
Reference
-
1. Ritossa F. A new puffing pattern induced by temperature and DNP in Drosophila. Experimentia. 1962. 18:571–573.2. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988. 22:631–677.
Article3. Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins. J Cell Sci. 2002. 115:2809–2816.
Article4. Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990. 248:850–854.
Article5. Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992. 12:2186–2192.
Article6. Nanbu K, Konish I, Mandai M, Kurosa H, Hamid AA, Komatsu T, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998. 22:549–555.
Article7. Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997. 33:873–877.
Article8. Jameel A, Skilton RA, Campbell TA, Chander SK, Coombes RC, Luqmani YA. Clinical and biological significance of HSP89 alpha in human breast cancer. Int J Cancer. 1992. 50:409–415.
Article9. Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998. 273:17147–17153.
Article10. Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000. 275:25665–25671.
Article11. Yufu Y, Nishimura J, Nawata H. High constitutive expression of heat shock protein 90 alpha in human acute leukemia cells. Leuk Res. 1992. 16:597–605.
Article12. Hsu PL, Hsu SM. Abundance of heat shock proteins (hsp89, hsp60, and hsp27) in malignant cells of Hodgkin's disease. Cancer Res. 1998. 58:5507–5513.13. Galea-Lauri J, Richardson AJ, Latchman DS, Katz DR. Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-alpha and cycloheximide: a possible role in immunopathology. J Immunol. 1996. 157:4109–4118.14. Pandey P, Saleh A, Nakazawa a, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. Embo J. 2000. 19:4310–4322.
Article15. Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002. 8:Suppl 4. 55–61.
Article16. Workman P. Pharmacogenomics in cancer drug discovery and development: inhibitors of the Hsp90 molecular chaperone. Cancer Detect Prev. 2002. 26:405–410.
Article17. Valcic S, TimmermannB ND, Alberts SG, Wachter A, Krutzsch M, Wymer J, et al. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anti-Cancer Drug. 1996. 7:461–468.
Article18. Singhal RL, Yeh YA, Praja N, Olah E, Sledge GW Jr, Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem Biophy Res Commun. 1995. 208:425–431.
Article19. Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, et al. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992. 12:3490–3498.
Article20. Larocca MF, Ranelletti O, Maggiano N, Rutella S, La Barbera EO, Rumi C, et al. Differential sensitivity of leukemic and normal hematopoietic progenitors to the killing effect of hyperthermia and quercetin used in combination: Role of heat-shock protein-70. Int J Cancer. 1997. 73:75–83.
Article21. Koishi M, Hosokawa N, Sato M, Nakai A, Hirayoshi K, Hiraoka M, et al. Quercetin, an inhibitor of heat shock protein synthesis, inhibits the acquisition of thermotolerance in a human colon carcinoma cell line. Jpn J Cancer Res. 1992. 3:1216–1222.
Article22. Yano M, Naito Z, Tanaka S, Asano G. Expression and roles of heat shock proteins in human breast cancer. Jpn J Cancer Res. 1996. 87:908–915.
Article23. Thanner F, Sutterlin MW, Kapp M, Rieger L, Kristen P, Dietl J, et al. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res. 2003. 23:1057–1062.24. Kiang JG, Gist ID, Tsokos GC. Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells. Mol Cell Biochem. 1999. 199:179–188.25. Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000. 2:469–475.
Article26. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000. 92:1564–1572.
Article27. Lewis J, Devin A, Miller A, Lin Y, Rodriquez Y, Neckers L, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000. 275:10519–10526.
Article28. Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy. A comprehensive review. Pharmacol Ther. 2004. 562:227–257.29. Hansen RK, Osterreich S, Lemieux P, Sarge KD, Fuqua SA. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem Biophys Res Comm. 1997. 239:851–856.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heat Shock Protein as Molecular Targets for Breast Cancer Therapeutics
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Understanding the Role of Heat Shock Protein Isoforms in Male Fertility, Aging and Apoptosis
- Heat Shock Protein Induction By An Infrared Warm Compression Device
- The Role of Heat Shock Protein in Perinatal Fields