Effects of Inositol 1,4,5-triphosphate on Osteoclast Differentiation in RANKL-induced Osteoclastogenesis
- Affiliations
-
- 1Department of Oral Biology, College of Dentistry, Yonsei University, Seoul 120-752, Korea. dmshin@yuhs.ac
- 2AMWAY Korea, Technical Service Division, Seoul 135-713, Korea.
- 3Yuhan Reaserach Institute, Seoul 135-725, Korea.
- KMID: 2285437
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.31
Abstract
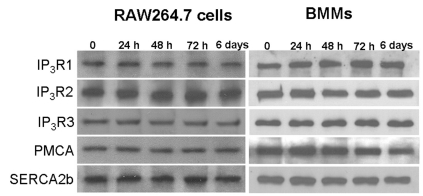
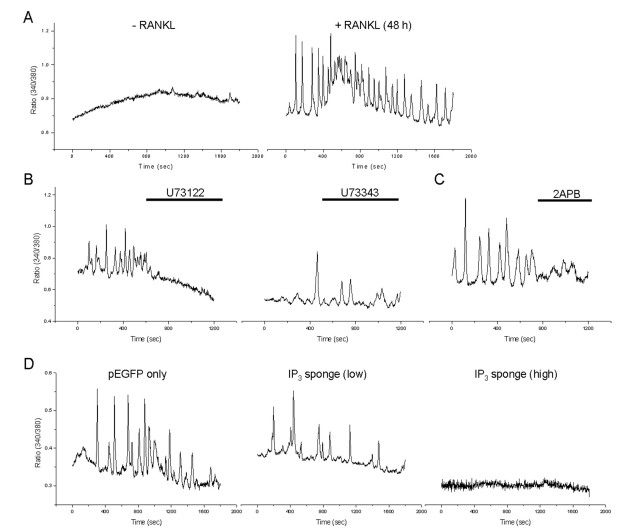
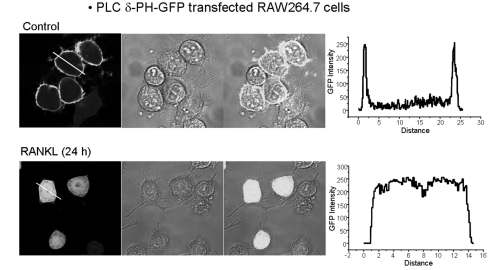
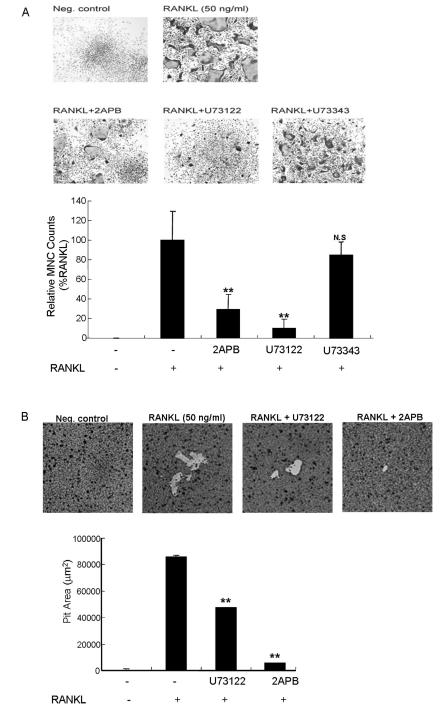
- The receptor activator of NF-kappaB ligand (RANKL) signal is an activator of tumor necrosis factor receptor-associated factor 6 (TRAF6), which leads to the activation of NF-kappaB and other signal transduction pathways essential for osteoclastogenesis, such as Ca2+ signaling. However, the intracellular levels of inositol 1,4,5-trisphosphate (IP3) and IP3-mediated cellular function of RANKL during osteoclastogenesis are not known. In the present study, we determined the levels of IP3 and evaluated IP3-mediated osteoclast differentiation and osteoclast activity by RANKL treatment of mouse leukemic macrophage cells (RAW 264.7) and mouse bone marrow-derived monocyte/macrophage precursor cells (BMMs). During osteoclastogenesis, the expression levels of Ca2+ signaling proteins such as IP3 receptors (IP3Rs), plasma membrane Ca2+ ATPase, and sarco/endoplasmic reticulum Ca2+ ATPase type2 did not change by RANKL treatment for up to 6 days in both cell types. At 24 h after RANKL treatment, a higher steady-state level of IP3 was observed in RAW264.7 cells transfected with green fluorescent protein (GFP)-tagged pleckstrin homology (PH) domains of phospholipase C (PLC) delta, a probe specifically detecting intracellular IP3 levels. In BMMs, the inhibition of PLC with U73122 [a specific inhibitor of phospholipase C (PLC)] and of IP3Rs with 2-aminoethoxydiphenyl borate (2APB; a non-specific inhibitor of IP3Rs) inhibited the generation of RANKL-induced multinucleated cells and decreased the bone-resorption rate in dentin slice, respectively. These results suggest that intracellular IP3 levels and the IP3-mediated signaling pathway play an important role in RANKL-induced osteoclastogenesis.
MeSH Terms
-
Animals
Blood Proteins
Boron Compounds
Calcium-Transporting ATPases
Cell Membrane
Dentin
Estrenes
Inositol
Inositol 1,4,5-Trisphosphate
Inositol 1,4,5-Trisphosphate Receptors
Macrophages
Mice
NF-kappa B
Osteoclasts
Phosphoproteins
Proteins
Pyrrolidinones
Receptor Activator of Nuclear Factor-kappa B
Reticulum
Signal Transduction
Tumor Necrosis Factor-alpha
Type C Phospholipases
Blood Proteins
Boron Compounds
Calcium-Transporting ATPases
Estrenes
Inositol
Inositol 1,4,5-Trisphosphate
Inositol 1,4,5-Trisphosphate Receptors
NF-kappa B
Phosphoproteins
Proteins
Pyrrolidinones
Receptor Activator of Nuclear Factor-kappa B
Tumor Necrosis Factor-alpha
Type C Phospholipases
Figure
Cited by 4 articles
-
Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
Namju Kang, Ki Woo Kim, Dong Min Shin
Korean J Physiol Pharmacol. 2019;23(5):411-417. doi: 10.4196/kjpp.2019.23.5.411.Activation of G Proteins by Aluminum Fluoride Enhances RANKL-Mediated Osteoclastogenesis
Boryung Park, Yu-Mi Yang, Byung-Jai Choi, Min Seuk Kim, Dong Min Shin
Korean J Physiol Pharmacol. 2013;17(5):427-433. doi: 10.4196/kjpp.2013.17.5.427.TRPM7 Is Essential for RANKL-Induced Osteoclastogenesis
Yu-Mi Yang, Hwi-Hoon Jung, Sung Jun Lee, Hyung-Jun Choi, Min Seuk Kim, Dong Min Shin
Korean J Physiol Pharmacol. 2013;17(1):65-71. doi: 10.4196/kjpp.2013.17.1.65.Bacterial PAMPs and Allergens Trigger Increase in [Ca2+]i-induced Cytokine Expression in Human PDL Fibroblasts
Ga-Yeon Son, Dong Min Shin, Jeong Hee Hong
Korean J Physiol Pharmacol. 2015;19(3):291-297. doi: 10.4196/kjpp.2015.19.3.291.
Reference
-
1. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21:115–137. PMID: 10782361.
Article2. Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007; 13:791–801. PMID: 17618270.
Article3. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001; 142:5050–5055. PMID: 11713196.
Article4. Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001; 20:1271–1280. PMID: 11250893.
Article5. Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl). 2005; 83:170–179. PMID: 15776286.
Article6. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342. PMID: 12748652.
Article7. Ha H, Kwak HB, Lee SK, Na DS, Rudd CE, Lee ZH, Kim HH. Membrane rafts play a crucial role in receptor activator of nuclear factor kappaB signaling and osteoclast function. J Biol Chem. 2003; 278:18573–18580. PMID: 12637570.8. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901. PMID: 12479813.
Article9. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article10. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004; 428:758–763. PMID: 15085135.
Article11. Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003; 162:293–303. PMID: 12860966.12. Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003; 15:243–253. PMID: 12531423.13. Uchiyama T, Yoshikawa F, Hishida A, Furuichi T, Mikoshiba K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP(3)) absorbent traps IP(3), resulting in specific inhibition of IP(3)-mediated calcium signaling. J Biol Chem. 2002; 277:8106–8113. PMID: 11741904.
Article14. Tian YS, Jeong HJ, Lee SD, Kong SH, Ohk SH, Yoo YJ, Seo JT, Shin DM, Sohn BW, Lee SI. Hyperosmotic Stimulus Down-regulates 1alpha, 25-dihydroxyvitamin D(3)-induced Osteoclastogenesis by Suppressing the RANKL Expression in a Co-culture System. Korean J Physiol Pharmacol. 2010; 14:169–176. PMID: 20631890.15. Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010; 285:6913–6921. PMID: 20048168.16. Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999; 284:1527–1530. PMID: 10348740.17. Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci USA. 2008; 105:8643–8648. PMID: 18552177.18. van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA. 2005; 102:17507–17512. PMID: 16291808.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
- OPG Inhibits PMMA Induced Osteoclastogenesis and NF-kappaB Activation in Osteoclast Precursor Cells
- Regulation of Osteoclast Differentiation: Identification of osteoclast and macrophage fusion protein; DC-STAMP
- The Molecular Mechanism of Baicalin on RANKL-induced Osteoclastogenesis in RAW264.7 Cells
- Adseverin mediates RANKL-induced osteoclastogenesis by regulating NFATc1