Allergy Asthma Immunol Res.
2013 Sep;5(5):329-336. 10.4168/aair.2013.5.5.329.
Suppression of Heme Oxygenase-1 by Prostaglandin E2-Protein Kinase A-A-Kinase Anchoring Protein Signaling Is Central for Augmented Cyclooxygenase-2 Expression in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages
- Affiliations
-
- 1Department of Internal Medicine, Eulji Hospital, Eulji University School of Medicine, Seoul, Korea. ksh1134@eulji.ac.kr
- 2Department of Bio-Medical Laboratory Science, College of Health Science, Eulji University, Suongnam, Korea.
- KMID: 2260815
- DOI: http://doi.org/10.4168/aair.2013.5.5.329
Abstract
- PURPOSE
Prostaglandin (PG) E2 is an immunomodulatory lipid mediator generated mainly via the cyclooxygenase-2 (COX-2) pathway from arachidonic acid at sites of infection and inflammation. A positive feedback loop of PGE2 on COX-2 expression is critical for homeostasis during toll-like receptor (TLR)-mediated inflammatory processes. The mechanism of PGE2-regulated COX-2 expression remains poorly understood. The low-molecular-weight stress protein heme oxygenase-1 (HO-1) contributes to the anti-inflammatory, anti-oxidant and anti-apoptotic response against environmental stress.
METHODS
We explored the involvement of HO-1 on PGE2 regulation of LPS-induced COX-2 expression in RAW 264.7 macrophages.
RESULTS
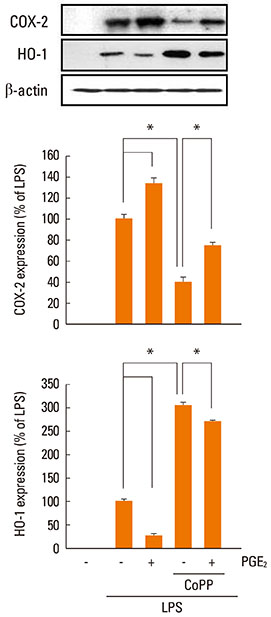
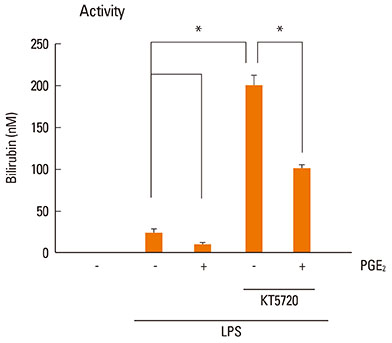
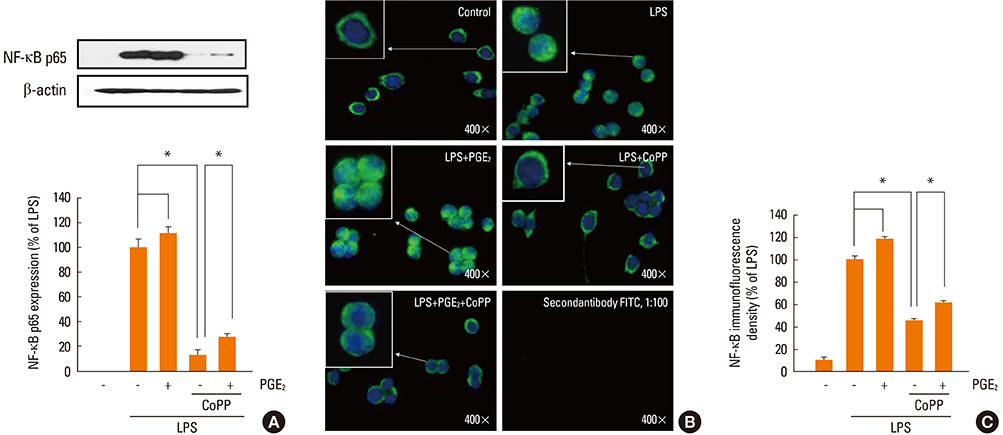
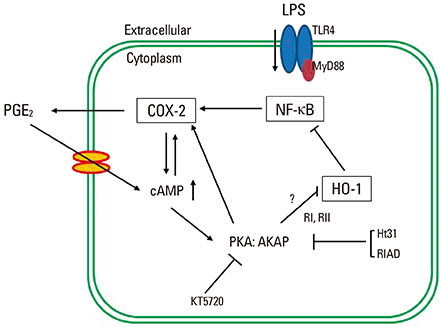
LPS-induced COX-2 expression in RAW 264.7 macrophages was enhanced by exogenous PGE2 or cyclic AMP (cAMP) analogue and was suppressed by a COX inhibitor (indomethacin), a protein kinase A (PKA) inhibitor (KT5720), and A kinase anchoring protein (AKAP) disruptors (Ht31 and RIAD). This result suggests that the stimulatory effects of endogenous and exogenous PGE2 on COX-2 expression are mediated by a cAMP-PKA-AKAP-dependent pathway. The induction of HO-1 was observed in LPS-stimulated RAW 264.7 macrophages. This induction was suppressed by exogenous PGE2 and enhanced by blockage of the endogenous PGE2 effect by the PKA inhibitor or AKAP disruptors. In addition, HO-1 induction by the HO activator copper protoporphyrin suppressed LPS-induced COX-2 expression, which was restored by the addition of exogenous PGE2. The induction of HO-1 inhibited LPS-induced NF-kappaB p-65 nuclear expression and translocation.
CONCLUSIONS
AKAP plays an important role in PGE2 regulation of COX-2 expression, and the suppression of HO-1 by PGE2-cAMP-PKA-AKAP signaling helps potentiate the LPS-induced COX-2 expression through a positive feedback loop in RAW 264.7 macrophages.
MeSH Terms
-
Arachidonic Acid
Copper
Cyclic AMP
Cyclic AMP-Dependent Protein Kinases
Cyclooxygenase 2
Dinoprostone
Heme
Heme Oxygenase-1
Homeostasis
Inflammation
Intracellular Signaling Peptides and Proteins
Macrophages
NF-kappa B
Phosphotransferases
Toll-Like Receptors
Arachidonic Acid
Copper
Cyclic AMP
Cyclic AMP-Dependent Protein Kinases
Cyclooxygenase 2
Dinoprostone
Heme
Heme Oxygenase-1
Intracellular Signaling Peptides and Proteins
NF-kappa B
Phosphotransferases
Toll-Like Receptors
Figure
Reference
-
1. Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB J. 2001; 15:2556–2564.2. Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009; 17:55–67.3. Ren W, Hu L, Hua F, Jin J, Wang Y, Zhu L. Myeloid differentiation protein 2 silencing decreases LPS-induced cytokine production and TLR4/MyD88 pathway activity in alveolar macrophages. Immunol Lett. 2011; 141:94–101.4. Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004; 25:40–46.5. Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008; 39:127–132.6. Jarnaess E, Taskén K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007; 35:931–937.7. Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006; 31:680–686.8. Hinz B, Brune K, Pahl A. Prostaglandin E(2) upregulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2000; 272:744–748.9. Díaz-Muñoz MD, Osma-García IC, Fresno M, Iñiguez MA. Involvement of PGE2 and the cAMP signalling pathway in the upregulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. Biochem J. 2012; 443:451–461.10. Hinz B, Brune K, Pahl A. Cyclooxygenase-2 expression in lipopolysaccharide-stimulated human monocytes is modulated by cyclic AMP, prostaglandin E(2), and nonsteroidal anti-inflammatory drugs. Biochem Biophys Res Commun. 2000; 278:790–796.11. Steinert D, Küper C, Bartels H, Beck FX, Neuhofer W. PGE2 potentiates tonicity-induced COX-2 expression in renal medullary cells in a positive feedback loop involving EP2-cAMP-PKA signaling. Am J Physiol Cell Physiol. 2009; 296:C75–C87.12. Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010; 80:1895–1903.13. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006; 86:583–650.14. Chin BY, Otterbein LE. Carbon monoxide is a poison... to microbes! CO as a bactericidal molecule. Curr Opin Pharmacol. 2009; 9:490–500.15. Suh GY, Jin Y, Yi AK, Wang XM, Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006; 35:220–226.16. Shih RH, Yang CM. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J Neuroinflammation. 2010; 7:86.17. Leung PO, Wang SH, Lu SH, Chou WH, Shiau CY, Chou TC. Simvastatin inhibits pro-inflammatory mediators through induction of heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264.7 macrophages. Toxicol Lett. 2011; 207:159–166.18. Waltz P, Carchman EH, Young AC, Rao J, Rosengart MR, Kaczorowski D, Zuckerbraun BS. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy. 2011; 7:315–320.19. Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000; 275:34035–34040.20. Chhikara M, Wang S, Kern SJ, Ferreyra GA, Barb JJ, Munson PJ, Danner RL. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-kappaB signal transduction in human monocytes. PLoS One. 2009; 4:e8139.21. Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009; 2:ra28.22. Kim SH, Serezani CH, Okunishi K, Zaslona Z, Aronoff DM, Peters-Golden M. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of Toll-like receptor signaling in alveolar macrophages. J Biol Chem. 2011; 286:8875–8883.23. Olszanecki R, Kurnyta M, Biedroń R, Chorobik P, Bereta M, Marcinkiewicz J. The role of heme oxygenase-1 in down regulation of PGE2 production by taurine chloramine and taurine bromamine in J774.2 macrophages. Amino Acids. 2008; 35:359–364.24. Liu XH, Pan LL, Yang HB, Gong QH, Zhu YZ. Leonurine attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: involvement of reactive oxygen species and NF-kappaB pathways. Eur J Pharmacol. 2012; 680:108–114.25. Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat heme oxygenase-1 gene is transcriptionally induced via the protein kinase A signaling pathway in rat hepatocyte cultures. Mol Pharmacol. 1998; 53:483–491.26. Kim HJ, Tsoy I, Park MK, Lee YS, Lee JH, Seo HG, Chang KC. Iron released by sodium nitroprusside contributes to heme oxygenase-1 induction via the cAMP-protein kinase A-mitogen-activated protein kinase pathway in RAW 264.7 cells. Mol Pharmacol. 2006; 69:1633–1640.27. Park MK, Kang YJ, Ha YM, Jeong JJ, Kim HJ, Seo HG, Lee JH, Chang KC. EP2 receptor activation by prostaglandin E2 leads to induction of HO-1 via PKA and PI3K pathways in C6 cells. Biochem Biophys Res Commun. 2009; 379:1043–1047.28. Nakao S, Ogata Y, Shimizu-Sasaki E, Yamazaki M, Furuyama S, Sugiya H. Activation of NFkappaB is necessary for IL-1beta-induced cyclooxygenase-2 (COX-2) expression in human gingival fibroblasts. Mol Cell Biochem. 2000; 209:113–118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- TI-I-174, a Synthetic Chalcone Derivative, Suppresses Nitric Oxide Production in Murine Macrophages via Heme Oxygenase-1 Induction and Inhibition of AP-1
- Anti-inflammatory effect of methanol extract from Erigeron Canadensis L. may be involved with upregulation of heme oxygenase-1 expression and suppression of NFkappaB and MAPKs activation in macrophages
- Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells
- Black soybean anthocyanins attenuate inflammatory responses by suppressing reactive oxygen species production and mitogen activated protein kinases signaling in lipopolysaccharide-stimulated macrophages