Allergy Asthma Immunol Res.
2014 Nov;6(6):567-572. 10.4168/aair.2014.6.6.567.
Histamine Promotes the Release of Interleukin-6 via the H1R/p38 and NF-kappaB Pathways in Nasal Fibroblasts
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. lhman@korea.ac.kr
- 2Department of Biomedical Sciences, Korea University Graduate School, Seoul, Korea.
- 3Medical Devices Clinical Trial Center, Guro Hospital, Korea University, Seoul, Korea.
- KMID: 2260180
- DOI: http://doi.org/10.4168/aair.2014.6.6.567
Abstract
- PURPOSE
Based on the close relationship between histamine and interleukin 6 (IL-6), we hypothesized that histamine may regulate the production of cytokines, such as IL-6, during allergic inflammation. Here, we examined the role of histamine in IL-6 production and histamine receptor activity in nasal fibroblasts, along with the mechanisms underlying these effects.
METHODS
Experiments were performed using nasal fibroblasts from 8 normal patients. RT-PCR was used to identify the major histamine receptors expressed in nasal fibroblasts. Fibroblasts were then treated with histamine with or without histamine-receptor antagonists, and monitored for IL-6 production using an ELISA. Four potential downstream signaling molecules, p38, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and NF-kappaB, were evaluated by Western blot, and a luciferase reporter assay.
RESULTS
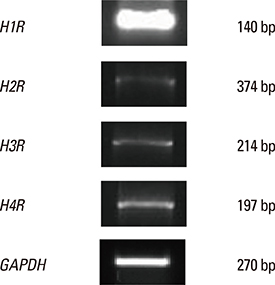
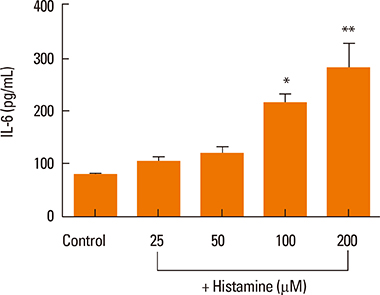
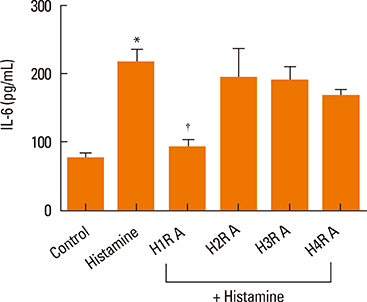
Elevated expression was seen for all histamine receptors, with IL-6 protein levels increasing significantly following histamine stimulation. Among the histamine-receptor specific antagonists, only the H1R antagonist significantly decreased IL-6 production in histamine-stimulated nasal fibroblasts. Histamine increased the expression level of phosphorylated p38 (pp38), pERK, and pJNK, as well as NF-kappaB induction. The H1R antagonist actively suppressed pp38 and NF-kappaB expression in histamine-induced nasal fibroblasts, but not pERK and pJNK. The p38 inhibitor strongly attenuated IL-6 production in histamine-stimulated nasal fibroblasts.
CONCLUSIONS
The data presented here suggest that antihistamines may be involved in the regulation of cytokines, such as IL-6, due to the role of histamine as an inflammatory mediator in nasal fibroblasts.
Keyword
MeSH Terms
-
Blotting, Western
Cytokines
Enzyme-Linked Immunosorbent Assay
Fibroblasts*
Histamine Antagonists
Histamine*
Humans
Inflammation
Interleukin-6*
JNK Mitogen-Activated Protein Kinases
Luciferases
NF-kappa B*
Nose
Phosphotransferases
Receptors, Histamine
Cytokines
Histamine
Histamine Antagonists
Interleukin-6
JNK Mitogen-Activated Protein Kinases
Luciferases
NF-kappa B
Phosphotransferases
Receptors, Histamine
Figure
Reference
-
1. Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000; 55:Suppl 64. 7–16.2. Mygind N, Secher C, Kirkegaard J. Role of histamine and antihistamines in the nose. Eur J Respir Dis Suppl. 1983; 128:16–20.3. Shirasaki H, Kanaizumi E, Seki N, Himi T. Localization and upregulation of the nasal histamine H1 receptor in perennial allergic rhinitis. Mediators Inflamm. 2012; 2012:951316.4. Rotrosen D, Gallin JI. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986; 103:2379–2387.5. Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993; 54:1–78.6. Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FE. Middleton's allergy: principles and practice. 7th ed. New York (NY): Mosby/Elsevier;2009.7. Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A. 1998; 95:8222–8226.8. Doganci A, Sauer K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005; 28:257–270.9. Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18, and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10, and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001; 125:177–183.10. Triggiani M, Gentile M, Secondo A, Granata F, Oriente A, Taglialatela M, Annunziato L, Marone G. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol. 2001; 166:4083–4091.11. Delneste Y, Lassalle P, Jeannin P, Joseph M, Tonnel AB, Gosset P. Histamine induces IL-6 production by human endothelial cells. Clin Exp Immunol. 1994; 98:344–349.12. Denburg JA, Gauldie J, Dolovich J, Ohtoshi T, Cox G, Jordana M. Structural cell-derived cytokines in allergic inflammation. Int Arch Allergy Appl Immunol. 1991; 94:127–132.13. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993; 328:257–265.14. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010; 125:S73–S80.15. Repka-Ramirez MS, Baraniuk JN. Histamine in health and disease. Clin Allergy Immunol. 2002; 17:1–25.16. Falus A, Merétey K. Histamine: an early messenger in inflammatory and immune reactions. Immunol Today. 1992; 13:154–156.17. Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998; 161:2586–2593.18. Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/IκB/NF-κB signal cascades. Biochem Pharmacol. 2005; 69:433–449.19. Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004; 113:43–49.20. Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003; 282:90–100.21. Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1α, IL-1β, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1α and TNF-α. Clin Exp Immunol. 1994; 96:437–443.22. Denburg JA, Dolovich J, Ohtoshi T, Cox G, Gauldie J, Jordana M. The microenvironmental differentiation hypothesis of airway inflammation. Am J Rhinol. 1990; 4:29–32.23. Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunasawa S, Sakiyama F. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986; 324:73–76.24. Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010; 22:347–352.25. Bachert C, Hauser U, Prem B, Rudack C, Ganzer U. Proinflammatory cytokines in allergic rhinitis. Eur Arch Otorhinolaryngol. 1995; 252:Suppl 1. S44–S49.26. Gosset P, Malaquin F, Delneste Y, Wallaert B, Capron A, Joseph M, Tonnel AB. Interleukin-6 and interleukin-1α production is associated with antigen-induced late nasal response. J Allergy Clin Immunol. 1993; 92:878–890.27. Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002; 39:531–536.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histamine Induced Production of Chemokine CXCL8 Through H1R/PLC and NF-κB Signaling Pathways in Nasal Fibroblasts
- Blockade of p38 Mitogen-activated Protein Kinase Pathway Inhibits Interleukin-6 Release and Expression in Primary Neonatal Cardiomyocytes
- Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-kappaB Pathways in Nasal Polyp-Derived Fibroblasts
- Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-kappaB, and MAPK Pathways
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways