J Breast Cancer.
2009 Sep;12(3):134-141. 10.4048/jbc.2009.12.3.134.
Reactive Oxygen Species Generated by 17beta-estradiol Play a Role in the Up-regulation of GPX4 Protein in MCF-7 Breast Cancer Cells
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- 2Department of Clinical Parasitology and Allergy, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- 3Department of Oncology and Department of Radiation Medicine, Lombardi Comprehensive Cancer Center, Georgetown University, Washington DC, USA. ib42@georgetown.edu
- KMID: 2242003
- DOI: http://doi.org/10.4048/jbc.2009.12.3.134
Abstract
- PURPOSE
Estrogen is known to act as both a growth factor and a survival factor for breast cancer. The responsible molecular mechanisms remain, however, to be fully elucidated. We hypothesize that the effect of estrogen relates to its ability to induce the cellular antioxidant defense enzymes.
METHODS
In the presence study, we examined the ability of 17beta-estradiol (E2) to regulate the level of phospholipid hydroperoxide glutathione peroxidase (GPX4) protein, which is an anti-oxidative enzyme that can directly reduce both phospholipids and cholesterol-hydroperoxides located in the cell membranes and lipoproteins.
RESULTS
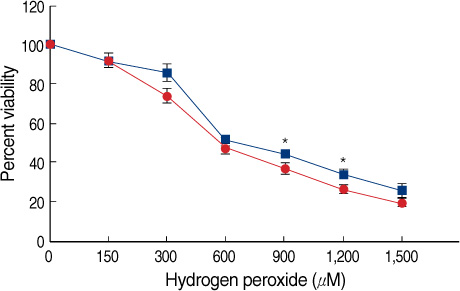
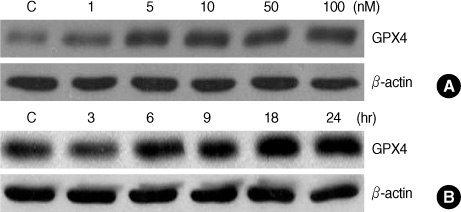
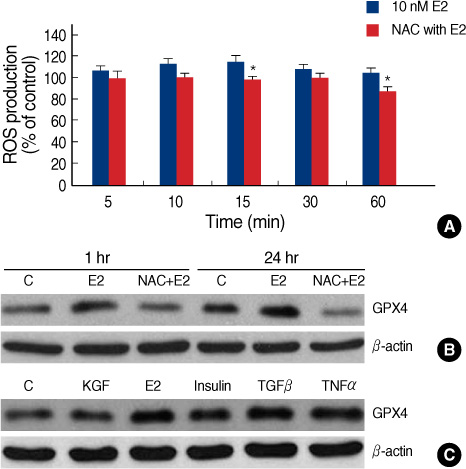
E2 elicited a dose- and time-dependent increase in the GPX4 expression in the MCF-7 breast cancer cells, and this up-regulation was blocked by the free radical scavenger N-acetylcysteine (NAC). Additionally, we confirmed that E2 triggered a rapid and transient increase in the intracellular reactive oxygen species (ROS) levels, and this E2-induced increase in the ROS levels was inhibited by pretreatment with NAC. Moreover, such ROS inducers as TGF-beta, TNF-alpha and insulin induced an increase in the level of GPX4 protein. However, estrogen receptor (ER)alpha knockdown by transfection with ERalpha-siRNA did not significantly change the GPX4 protein level that was induced by E2. Furthermore, pre-incubation with the ER antagonist ICI 182,780 did not inhibit E2-mediated GPX4 induction. Conversely, pretreatment of cells with LY294002, a pharmacological inhibitor of phosphatidylinositol 3-kinase inhibitor, suppressed the E2-augmented GPX4 expression.
CONCLUSION
Collectively, our data show that E2 may partly provide a survival advantage through the regulation of cellular oxidative homeostasis in MCF-7 breast cancer cells.
Keyword
MeSH Terms
-
Acetylcysteine
Breast
Breast Neoplasms
Cell Membrane
Chromones
Estradiol
Estrogens
Glutathione Peroxidase
Homeostasis
Hydrogen Peroxide
Imidazoles
Insulin
Lipoproteins
Morpholines
Nitro Compounds
Oxidative Stress
Phosphatidylinositol 3-Kinase
Phospholipids
Reactive Oxygen Species
Receptors, Estrogen
Transfection
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Up-Regulation
Acetylcysteine
Chromones
Estradiol
Estrogens
Glutathione Peroxidase
Hydrogen Peroxide
Imidazoles
Insulin
Lipoproteins
Morpholines
Nitro Compounds
Phosphatidylinositol 3-Kinase
Phospholipids
Reactive Oxygen Species
Receptors, Estrogen
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the nurses' health study. N Engl J Med. 1991. 325:756–762.
Article2. Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, et al. Oestrogen for Alzheimer's diseases in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000. 54:295–301.
Article3. Ruiz-Larrea MB, Leal AM, Martín C, Martínez R, Lacort M. Antioxidant action of estrogens in rat hepatocytes. Rev Esp Fisiol. 1997. 53:225–229.4. Arai M, Imai H, Koumura T, Yoshida M, Emoto K, Umeda M, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J Biol Chem. 1999. 274:4924–4933.
Article5. Lippman ME, Bates S, Huff KK, Davidson N, Dickson RB. Estrogens regulate production of specific growth factors in hormone-dependent human breast cancer. J Lab Clin Med. 1987. 109:230–235.
Article6. Fernando RI, Wimalasena J. Estradiol abrogates apoptosis in breast cancer cells through inactivation of BAD: Ras-dependent nongenomic pathways requiring signaling through ERK and Akt. Mol Biol Cell. 2004. 15:3266–3284.
Article7. Huang Y, Ray S, Reed JC, Ibrado AM, Tang C, Nawabi A, et al. Estrogen increases intracellular p26Bcl-2 to p21Bax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1997. 42:73–81.
Article8. Persky AM, Green PS, Stubley L, Howell CO, Zaulyanov L, Brazeau GA, et al. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000. 223:59–66.
Article9. Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001. 22:33–66.
Article10. Ruiz-Larrea MB, Garrido MJ, Lacort M. Estradiol-induced effects on glutathione metabolism in rat hepatocytes. J Biochem. 1993. 113:563–567.11. Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol Endocrinol. 2008. 22:1113–1124.
Article12. Felty Q, Singh KP, Roy D. Estrogen-induced G1/S transition of G0-arrested estrogen-dependent breast cancer cells is regulated by mitochondrial oxidant signaling. Oncogene. 2005. 24:4883–4893.
Article13. Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999. 48:41–47.
Article14. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002. 82:47–95.
Article15. Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994. 126:1079–1088.
Article16. Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998. 39:1529–1542.
Article17. Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999. 13:295–309.18. Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003. 34:145–169.
Article19. Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ 2nd, et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004. 279:55137–55146.
Article20. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999. 27:612–616.
Article21. Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, et al. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005. 44:6900–6909.
Article22. Parkash J, Felty Q, Roy D. Estrogen exerts a spatial and temporal influence on reactive oxygen species generation that precedes calcium uptake in high-capacity mitochondria: implications for rapid nongenomic signaling of cell growth. Biochemistry. 2006. 45:2872–2881.
Article23. Miura T, Muraoka S, Ogiso T. Inhibition of lipid peroxidation by estradiol and 2-hydroxyestradiol. Steroids. 1996. 61:379–383.
Article24. Tang M, Subbiah MT. Estrogens protect against hydrogen peroxide and arachidonic acid induced DNA damage. Biochim Biophys Acta. 1996. 1299:155–159.
Article25. Ejima K, Nanri H, Araki M, Uchida K, Kashimura M, Ikeda M. 17beta-estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol. 1999. 140:608–613.
Article26. Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem. 1999. 274:29294–29302.
Article27. Huang HS, Chang WC, Chen CJ. Involvement of reactive oxygen species in arsenite-induced downregulation of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. Free Radic Biol Med. 2002. 33:864–873.
Article28. Chen ZH, Yoshida Y, Saito Y, Niki E. Adaptation to hydrogen peroxide enhances PC12 cell tolerance against oxidative damage. Neurosci Lett. 2005. 383:256–259.
Article29. Jarrett SG, Boulton ME. Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells. Free Radic Biol Med. 2005. 38:1382–1391.
Article30. Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17beta protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008. 18:491–499.
Article31. Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, et al. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000. 275:10527–10531.
Article32. Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002. 277:3101–3108.
Article33. Qin S, Chock PB. Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry. 2003. 42:2995–3003.
Article34. Tu VC, Bahl JJ, Chen QM. Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther. 2002. 300:1101–1110.
Article35. So MC, Hwang HP, Lee CH, Youn HJ, Jung SH, Kim JC. Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha in breast cancer cells. J Breast Cancer. 2006. 9:91–97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells
- Up-regulation of P13K/Akt Signaling by 17 beta-estradiol through Activation of Estrogen Receptor-alpha in Breast Cancer Cells
- Mutational Analysis of 17beta-hydroxysteroid dehydrogenase type 2 gene in Breast Cancers
- Effects of Polyamines on TNFalpha- or Tamoxifen-induced Apoptosis in Human Breast Cancer Cells
- Microgel-Encapsulated Methylene Blue for the Treatment of Breast Cancer Cells by Photodynamic Therapy