Korean Circ J.
2009 Aug;39(8):328-334. 10.4070/kcj.2009.39.8.328.
Metalloproteinase-3 Genotype as a Predictor of Cardiovascular Risk in Hypertensive Adolescents
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea. hongym@chollian.net
- KMID: 2225683
- DOI: http://doi.org/10.4070/kcj.2009.39.8.328
Abstract
- BACKGROUND AND OBJECTIVES
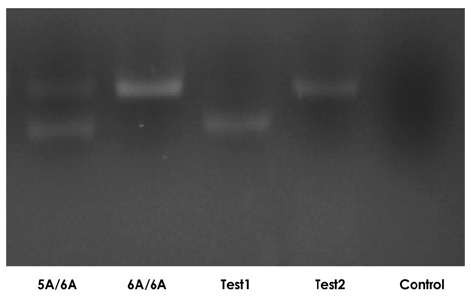
Hypertension develops as a result of cardiac hypertrophy and fibrosis or as a result of exchange of the extracellular matrix. In particular, matrix metalloproteinase (MMP)-3 is a major enzyme involved in the reconstruction of the arterial intima through activation of other MMPs. We analyzed MMP-3 genotypes in hypertensive and normotensive adolescents and sought to determine if a particular genotype is a predictor of cardiovascular complications. SUBJECTS AND METHODS: Forty-four hypertensive adolescents and 59 healthy adolescents were included in this study. Serum aldosterone, renin, insulin, angiotensin converting enzyme (ACE), insulin, homocysteine, vitamin B12, folate, MMP-1, MMP-2, MMP-3, MMP-9, tissue inhibitors of matrix metalloproteinases (TIMP)-1, and TIMP-2 were measured. MMP-3 genotypes were analyzed using a polymerase chain reaction (PCR) primer. The carotid intima media thickness (IMT), diameter, and brachial ankle pulse wave velocity (baPWV) were evaluated using ultrasound. RESULTS: In hypertensive adolescents, blood pressure, anthropometric data, carotid IMT, baPWV, serum pro-MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 were no different between the 6A/6A group and the 5A/6A group. Serum MMP-9 was higher in the 5A/6A group than in the control group. Aldosterone, insulin, and homocysteine were higher in the 6A/6A group than in the control group, and vitamin B12 and folate were lower in the 6A/6A group than in the control group. CONCLUSION: In conclusion, serum MMP-3 levels were not significantly different in different MMP-3 genotypes in hypertensive adolescents. However, few patients were included in this study. Further investigation is necessary to clarify the relationship between MMP-3 genotype and cardiovascular risk.
Keyword
MeSH Terms
-
Adolescent
Aldosterone
Animals
Ankle
Blood Pressure
Cardiomegaly
Carotid Intima-Media Thickness
Extracellular Matrix
Fibrosis
Folic Acid
Genotype
Homocysteine
Humans
Hypertension
Insulin
Matrix Metalloproteinases
Peptidyl-Dipeptidase A
Polymerase Chain Reaction
Pulse Wave Analysis
Renin
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Tunica Intima
Vitamin B 12
Aldosterone
Folic Acid
Homocysteine
Insulin
Matrix Metalloproteinases
Peptidyl-Dipeptidase A
Renin
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Vitamin B 12
Figure
Reference
-
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005. 365:217–223.2. Kim JM, Shin DJ, Bae YJ, et al. Association between I/D, G14480C, A22982G polymorphisms of angiotension I-converting enzyme gene and essential hypertension in the Korean population. Korean Circ J. 2004. 34:1137–1147.3. Lee JA, Sohn JA, Hong YM. Polymorphism of angiotensin II type 1 receptor A1166C in Korean hypertensive adolescents. Korean Circ J. 2008. 38:405–410.4. Jalil JE, Doering CW, Janicki JS, Pick RZ, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989. 64:1041–1050.5. Bashey RI, Cox R, McCann J, Jimenez SA. Changes in collagen biosynthesis, types, and mechanics of aorta in hypertensive rats. J Lab Clin Med. 1989. 113:604–611.6. Laviades C, Vain N, Fernandez J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998. 98:535–540.7. Safar ME. Therapeutic trials and large arteries in hypertension. Am Heart J. 1988. 115:702–710.8. Dzau VJ, Safar ME. Large conduit arteries in hypertension: role of the renin-angiotensin system. Circulation. 1988. 77:947–954.9. Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006. 113:2089–2096.10. Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002. 2:657–672.11. Parks WE. A confederancy of proteinases. J Clin Invest. 2002. 110:613–614.12. Vu TH, Werbz Z. Matrix metalloprotienases: effectors of development and normal physiology. Genes Dev. 2000. 14:2123–2133.13. Karthikeyan VJ, Lip GY. Matrix metalloproteinases and hypertension: a link between left ventricular hypertrophy and diastolic function? Tohoku J Exp Med. 2006. 208:93–97.14. Saglam M, Karakaya O, Esen AM, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006. 208:117–122.15. Tayebjee MH, Nedar SK, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 levels in patients with hypertension: relationship to tissue Doppler indicies of diastolic relaxation. Am J Hypertens. 2004. 17:770–774.16. Tayebjee MH, Lim HS, Nadar S, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinases-I is a marker of diastolic dysfunction using tissue Doppler in patients with type 2 diabetes and hypertension. Eur J Clin Invest. 2005. 35:8–12.17. Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003. 17:119–124.18. Asano Y, Iwai S, Okazaki M, et al. Matrix metalloproteinase-9 in spontaneously hypertensive hyperlipidemic rats. Pathophysiology. 2008. 15:157–166.19. Yasmin , McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005. 25:372.20. Murphy G, Ward R, Gavrilovic J, Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992. 1:224–230.21. Seccia TM, Bettini E, Vukous V, et al. Extracellular matrix gene expression in the left ventricular tissue of spontaneously hypertensive rats. Blood Press. 1999. 8:57–64.22. Medley TL, Cole TJ, Dart AM, Gatzka CD, Kingwell BA. Matrix metalloproteinase-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol. 2004. 24:1479–1484.23. Rhee MY, Lee HY, Park JB. Measurements of arterial stiffness: methological aspects. Korean Circ J. 2008. 38:343–350.24. Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007. 30:959–963.25. Lin J, Davis HB, Dai Q, et al. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertens Res. 2008. 31:1225–1231.26. Ishikawa J, Kario K, Matsui Y, et al. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 2005. 28:995–1001.27. Armstrong C, Abilleira S, Sitzer M, Markus H, Bevan S. Polymorphsims in MMP family and TIMP genes and carotid artery intima-media thickness. Stroke. 2007. 38:2895–2899.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiotensinogen gene M235T polymorphism as a predictor of cardiovascular risk in hypertensive adolescents
- Serum level of the adiponectin and adiponectin I164T polymorphism in hypertensive adolescents
- Left Ventricular Hypertrophy and Prelude to Hypertensive Cardiovascular Diseases; from the Pediatric Cardiologist's Point of View
- Association between Serine/Threonine Kinase 39 Gene Polymorphism, Hypertension, and Other Cardiovascular Risk Factors in Koreans
- Polymorphism of the Angiotensin II Type 1 Receptor A1166C in Korean Hypertensive Adolescents