J Korean Surg Soc.
2013 Dec;85(6):249-260. 10.4174/jkss.2013.85.6.249.
Effect of quercetin on apoptosis of PANC-1 cells
- Affiliations
-
- 1Division of Hepatobiliary Surgery, Department of Surgery, Wonkwang University School of Medicine & Hospital, Iksan, Korea. chaekm@wonkwang.ac.kr
- KMID: 2212545
- DOI: http://doi.org/10.4174/jkss.2013.85.6.249
Abstract
- PURPOSE
To investigate the chemotherapeutic effect of quercetin against cancer cells, signaling pathway of apoptosis was explored in human pancreatic cells.
METHODS
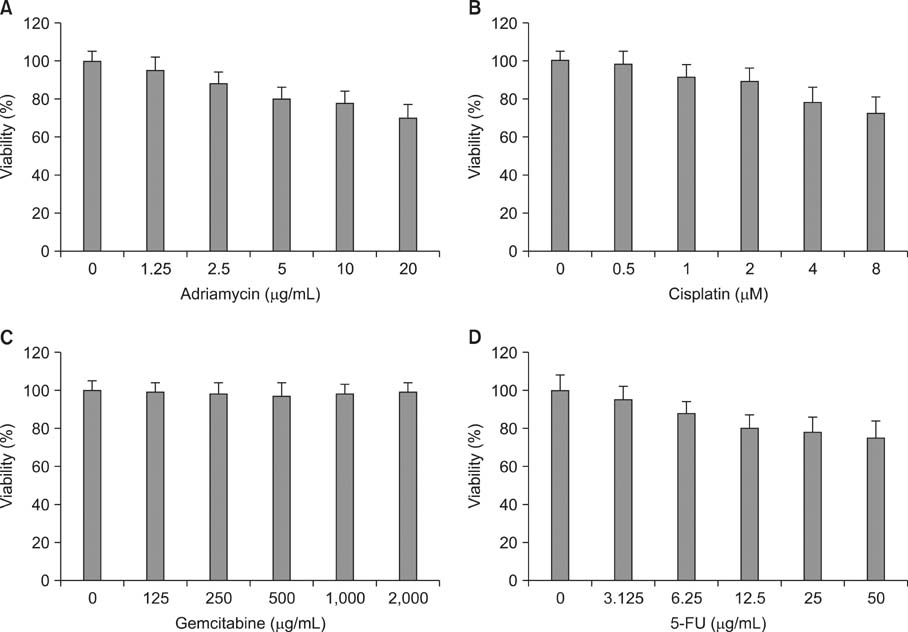
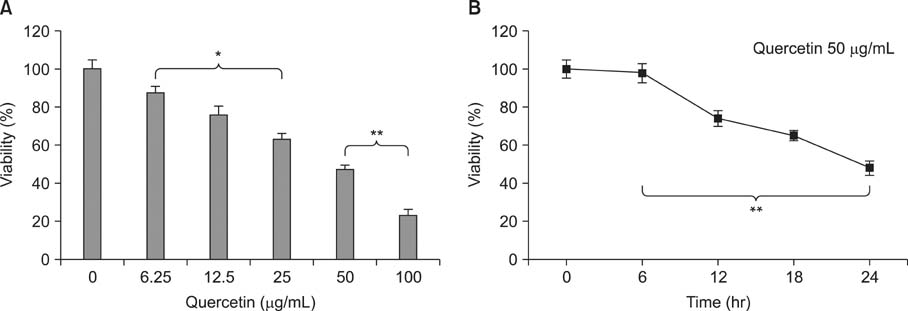
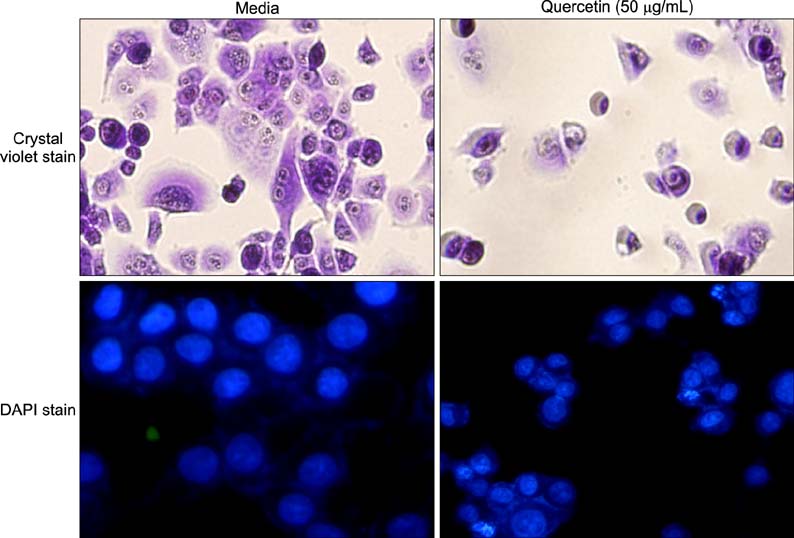
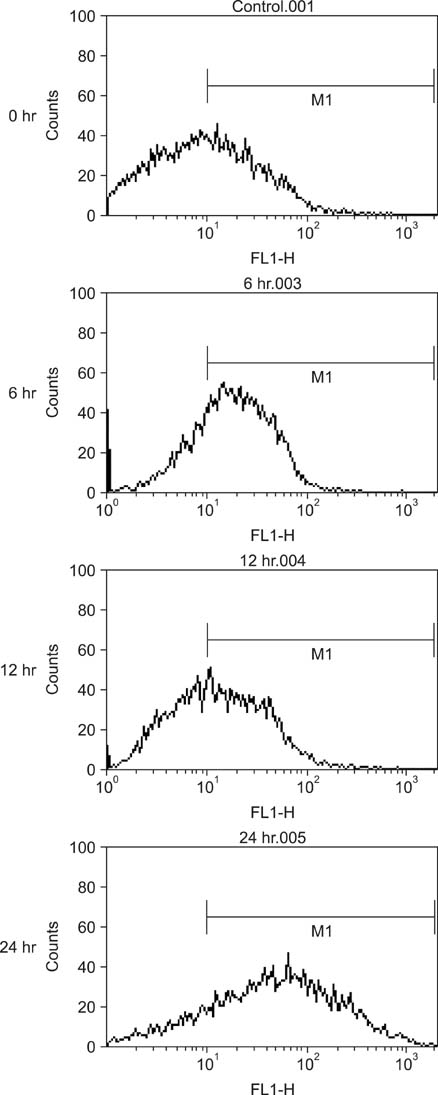
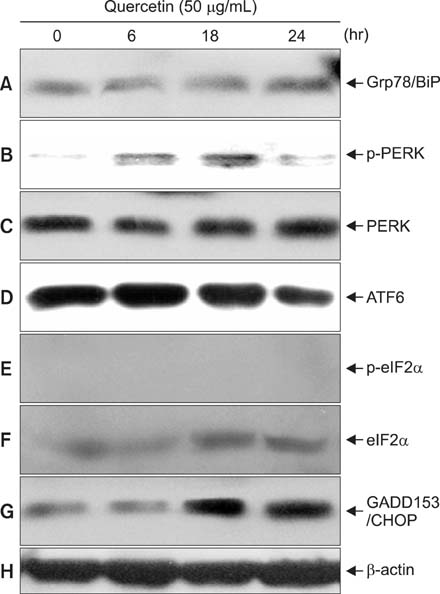
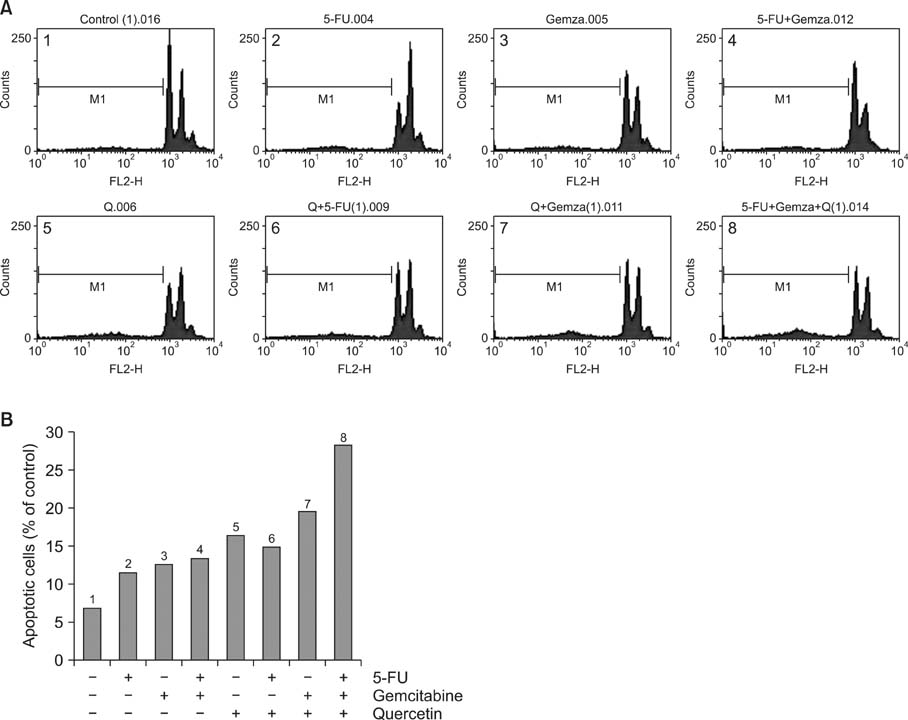
Various anticancer drugs including adriamycin, cisplatin, 5-fluorouracil (5-FU) and gemcitabine were used. Cell viability was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphe-nyltetra zolium bromide assay. Apoptosis was determined by 4'-6-diamidino-2-phenylindole nuclei staining and flow cytometry in PANC-1 cells treated with 50 microg/mL quercetin for 24 hours. Expression of endoplas mic reticulum (ER) stress mediators including, Grp78/Bip, p-PERK, PERK, ATF4, ATF6 and GADD153/CHOP proteins were measured by Western blot analysis. Mitochondrial membrane potential was measured by fluorescence staining with JC-1, rhodamine 123. Quercetin induced the apoptosis of PANC-1, which was characterized as nucleic acid and genomic DNA fragmentation, chromatin condensation, and sub-G0/G1 fraction of cell cycle increase. But not adriamycin, cisplatin, gemcitabine, and 5-FU. PANC-1 cells were markedly sensitive to quercetin.
RESULTS
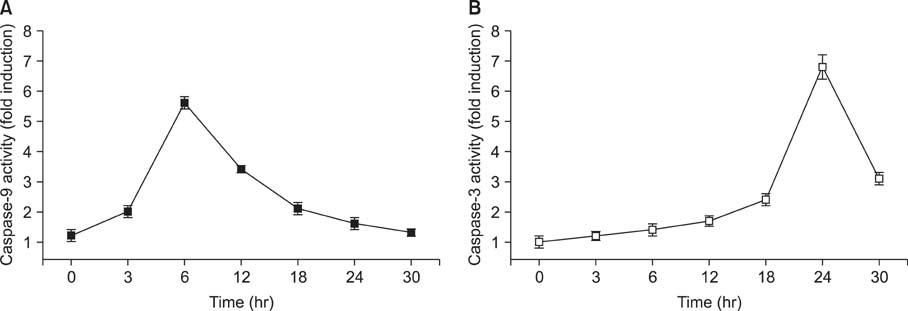
Treatment with quercetin resulted in the increased accumulation of intracellular Ca2+ ion. Treatment with quercetin also increased the expression of Grp78/Bip and GADD153/CHOP protein and induced mitochondrial dysfunction. Quercetin exerted cytotoxicity against human pancreatic cancer cells via ER stress-mediated apoptotic signaling including reactive oxygen species production and mitochondrial dysfunction.
CONCLUSION
These data suggest that quercetin may be an important modulator of chemosensitivity of cancer cells against anticancer chemotherapeutic agents.
Keyword
MeSH Terms
-
Apoptosis*
Benzimidazoles
Blotting, Western
Carbocyanines
Cell Cycle
Cell Survival
Chromatin
Cisplatin
Deoxycytidine
DNA Fragmentation
Doxorubicin
Drug Therapy
Flow Cytometry
Fluorescence
Fluorouracil
Humans
Membrane Potential, Mitochondrial
Pancreatic Neoplasms
Quercetin*
Reactive Oxygen Species
Reticulum
Rhodamine 123
Benzimidazoles
Carbocyanines
Chromatin
Cisplatin
Deoxycytidine
Doxorubicin
Fluorouracil
Quercetin
Reactive Oxygen Species
Rhodamine 123
Figure
Reference
-
1. Kim YT. Chemotherapy for pancreatic cancer. Korean J Gastroenterol. 2008; 51:111–118.2. Ekbom A, McLaughlin JK, Karlsson BM, Nyren O, Gridley G, Adami HO, et al. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994; 86:625–627.3. Mehmet H. Caspases find a new place to hide. Nature. 2000; 403:29–30.4. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000; 403:98–103.5. Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis: cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002; 277:34287–34294.6. Scambia G, Ranelletti FO, Panici PB, De Vincenzo R, Bonanno G, Ferrandina G, et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994; 34:459–464.7. Chen J, Kang JH. Quercetin and trichostatin A cooperatively kill human leukemia cells. Pharmazie. 2005; 60:856–860.8. Priego S, Feddi F, Ferrer P, Mena S, Benlloch M, Ortega A, et al. Natural polyphenols facilitate elimination of HT-29 colorectal cancer xenografts by chemoradiotherapy: a Bcl-2- and superoxide dismutase 2-dependent mechanism. Mol Cancer Ther. 2008; 7:3330–3342.9. Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996; 73:101–105.10. Searle J, Kerr JF, Bishop CJ. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982; 17(Pt 2):229–259.11. Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993; 262:695–700.12. Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990; 40:2353–2362.13. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999; 15:269–290.14. Hannun YA. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997; 89:1845–1853.15. Cohen JJ. Apoptosis: the physiologic pathway of cell death. Hosp Pract (Off Ed). 1993; 28:35–43.16. Rice-Evans CA, Miller NJ. Structure-antioxidant activity relationships of flavonoids and isoflavonoids. In : Rice-Evans CA, Packer L, editors. Flavonoids in health and disease. New York: Marcel Dekker;1998. p. 199–238.17. Edenharder R, Grunhage D. Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat Res. 2003; 540:1–18.18. Park C, So HS, Shin CH, Baek SH, Moon BS, Shin SH, et al. Quercetin protects the hydrogen peroxide-induced apoptosis via inhibition of mitochondrial dysfunction in H9c2 cardiomyoblast cells. Biochem Pharmacol. 2003; 66:1287–1295.19. Soloviev A, Stefanov A, Parshikov A, Khromov A, Moibenko A, Kvotchina L, et al. Arrhythmogenic peroxynitrite-induced alterations in mammalian heart contractility and its prevention with quercetin-filled liposomes. Cardiovasc Toxicol. 2002; 2:129–139.20. Kahraman A, Inal ME. Protective effects of quercetin on ultraviolet A light-induced oxidative stress in the blood of rat. J Appl Toxicol. 2002; 22:303–309.21. Mahesh T, Menon VP. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004; 18:123–127.22. Park C, So HS, Shin CH, Baek SH, Moon BS, Shin SH, et al. Quercetin protects the hydrogen peroxide-induced apoptosis via inhibition of mitochondrial dysfunction in H9c2 cardiomyoblast cells. Biochem Pharmacol. 2003; 66:1287–1295.23. Ferrari D, Stepczynska A, Los M, Wesselborg S, Schulze-Osthoff K. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J Exp Med. 1998; 188:979–984.24. Kaneko Y, Tsukamoto A. Thapsigargin-induced persistent intracellular calcium pool depletion and apoptosis in human hepatoma cells. Cancer Lett. 1994; 79:147–155.25. McCall CA, Cohen JJ. Programmed cell death in terminally differentiating keratinocytes: role of endogenous endonuclease. J Invest Dermatol. 1991; 97:111–114.26. Putney JW Jr. Calcium signaling: up, down, up, down... what's the point? Science. 1998; 279:191–192.27. Bellomo G, Perotti M, Taddei F, Mirabelli F, Finardi G, Nicotera P, et al. Tumor necrosis factor alpha induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res. 1992; 52:1342–1346.28. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002; 3:99–111.29. Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003; 4:265–271.30. Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999; 6:842–854.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Heat Shock Protein 70 Modulates the Chemoresponsiveness of Pancreatic Cancer
- Autophagy Inhibition Promotes Quercetin Induced Apoptosis in MG-63 Human Osteosarcoma cells
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Quercetin ameliorates glutamate toxicity-induced neuronal cell death by controlling calcium-binding protein parvalbumin
- Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity