J Bone Metab.
2016 Feb;23(1):8-15. 10.11005/jbm.2016.23.1.8.
Deficiency of Lipocalin-2 Promotes Proliferation and Differentiation of Osteoclast Precursors via Regulation of c-Fms Expression and Nuclear Factor-kappa B Activation
- Affiliations
-
- 1Department of Biomedical Science, Cell and Matrix Research Institute, BK21 Plus KNU Biomedical Convergence Program, Clinical Trial Center, School of Medicine, Kyungpook National University and Hospital, Daegu, Korea. biohjk@knu.ac.kr, yry@knu.ac.kr
- 2Skeletal Diseases Genome Research Center, School of Medicine, Kyungpook National University, Daegu, Korea.
- 3Department of Pharmacology, School of Medicine, Kyungpook National University, Daegu, Korea.
- 4College of Pharmacy, Yeungnam University, Gyeongsan, Korea.
- KMID: 2156281
- DOI: http://doi.org/10.11005/jbm.2016.23.1.8
Abstract
- BACKGROUND
Lipocalin-2 (LCN2), a small glycoprotein, has a pivotal role in diverse biological processes such as cellular proliferation and differentiation. We previously reported that LCN2 is implicated in osteoclast formation induced by receptor activator of nuclear factor-kappa B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). In the present study, we used a knockout mouse model to further investigate the role of LCN2 in osteoclast development.
METHODS
Osteoclastogenesis was assessed using primary bone marrow-derived macrophages. RANKL and M-CSF signaling was determined by immunoblotting, cell proliferation by bromodeoxyuridine (BrdU) enzyme-linked immunosorbent assay (ELISA), and apoptosis by cell death detection ELISA. Bone morphometric parameters were determined using a micro-computed tomography system.
RESULTS
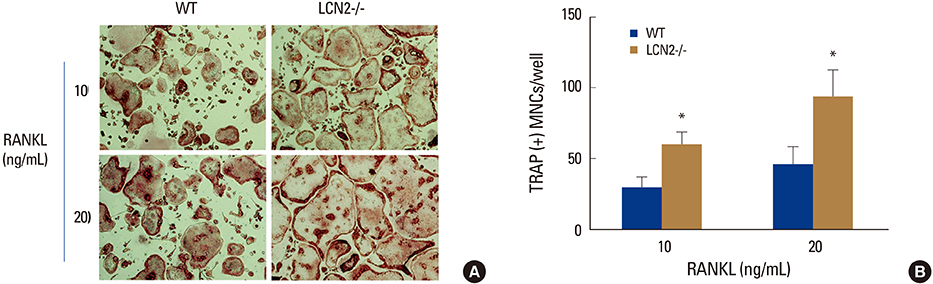
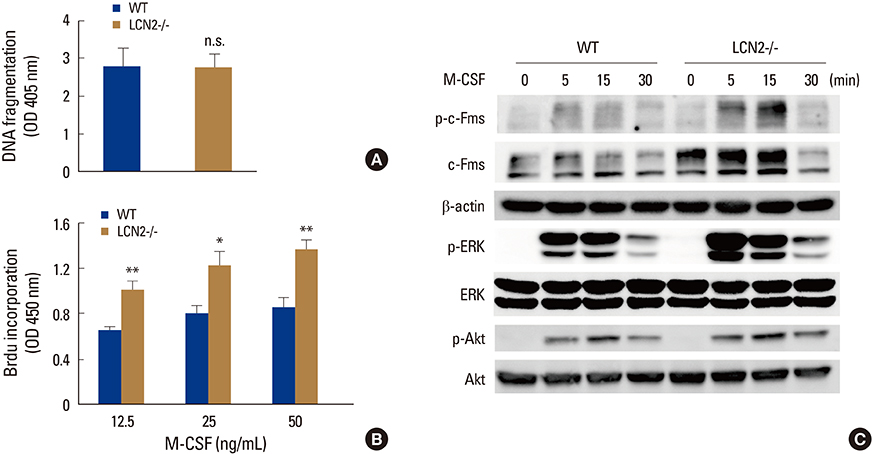
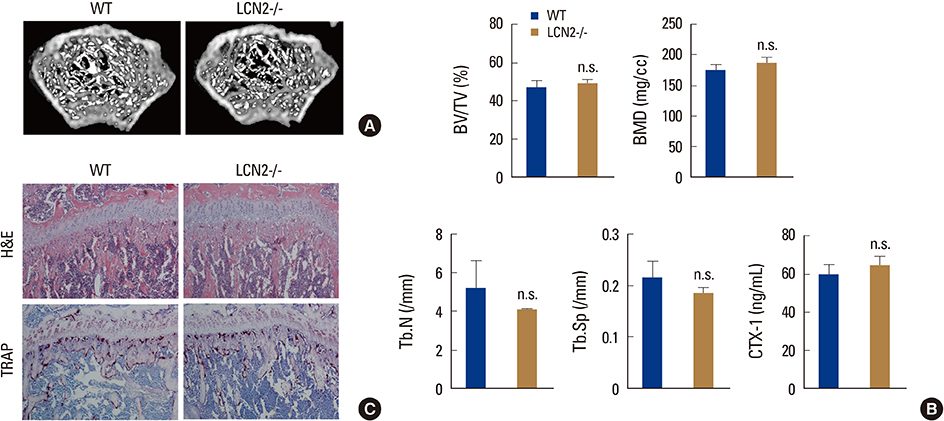
Our results showed that LCN2 deficiency increases tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclast formation in vitro, a finding that reflects enhanced proliferation and differentiation of osteoclast lineage cells. LCN2 deficiency promotes M-CSF-induced proliferation of bone marrow macrophages (BMMs), osteoclast precursors, without altering their survival. The accelerated proliferation of LCN2-deficient precursors is associated with enhanced expression and activation of the M-CSF receptor, c-Fms. Furthermore, LCN2 deficiency stimulates the induction of c-Fos and nuclear factor of activated T cells c1 (NFATc1), key transcription factors for osteoclastogenesis, and promotes RANKL-induced inhibitor of kappa B (IkappaBalpha) phosphorylation. Interestingly, LCN2 deficiency does not affect basal osteoclast formation in vivo, suggesting that LCN2 might play a role in the enhanced osteoclast development that occurs under some pathological conditions.
CONCLUSIONS
Our study establishes LCN2 as a negative modulator of osteoclast formation, results that are in accordance with our previous findings.
Keyword
MeSH Terms
-
Acid Phosphatase
Animals
Apoptosis
Biological Processes
Bone Marrow
Bromodeoxyuridine
Cell Death
Cell Proliferation
Enzyme-Linked Immunosorbent Assay
Glycoproteins
Immunoblotting
Macrophage Colony-Stimulating Factor
Macrophages
Mice
Mice, Knockout
NF-kappa B
Osteoclasts*
Phosphorylation
RANK Ligand
T-Lymphocytes
Transcription Factors
Acid Phosphatase
Bromodeoxyuridine
Glycoproteins
Macrophage Colony-Stimulating Factor
NF-kappa B
RANK Ligand
Transcription Factors
Figure
Cited by 1 articles
-
Fexaramine Inhibits Receptor Activator of Nuclear Factor-κB Ligand-induced Osteoclast Formation via Nuclear Factor of Activated T Cells Signaling Pathways
Ting Zheng, Na-Young Kim, Mijung Yim
J Bone Metab. 2017;24(4):207-215. doi: 10.11005/jbm.2017.24.4.207.
Reference
-
1. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article2. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649.
Article3. Jaworowski A, Wilson NJ, Christy E, et al. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J Biol Chem. 1999; 274:15127–15133.
Article4. Takeshita S, Faccio R, Chappel J, et al. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007; 282:18980–18990.
Article5. Valledor AF, Comalada M, Xaus J, et al. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000; 275:7403–7409.
Article6. Kelley TW, Graham MM, Doseff AI, et al. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999; 274:26393–26398.
Article7. Zhou P, Kitaura H, Teitelbaum SL, et al. SHIP1 negatively regulates proliferation of osteoclast precursors via Akt-dependent alterations in D-type cyclins and p27. J Immunol. 2006; 177:8777–8784.
Article8. Kim HJ, Zhao H, Kitaura H, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006; 116:2152–2160.
Article9. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.
Article10. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432:917–921.
Article11. Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002; 10:1033–1043.
Article12. Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006; 103:1834–1839.
Article13. Devireddy LR, Gazin C, Zhu X, et al. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005; 123:1293–1305.
Article14. Lee S, Lee J, Kim S, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007; 179:3231–3241.
Article15. Nelson AM, Zhao W, Gilliland KL, et al. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008; 118:1468–1478.
Article16. Miharada K, Hiroyama T, Sudo K, et al. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2008; 215:526–537.
Article17. Miharada K, Hiroyama T, Sudo K, et al. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005; 19:1881–1883.
Article18. Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008; 52:595–605.
Article19. Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002; 10:1045–1056.
Article20. Kim HJ, Yoon HJ, Yoon KA, et al. Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp Cell Res. 2015; 334:301–309.
Article21. Jeon S, Jha MK, Ock J, et al. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. J Biol Chem. 2013; 288:24116–24127.
Article22. Kim HJ, Yoon HJ, Choi JY, et al. The tyrosine kinase inhibitor GNF-2 suppresses osteoclast formation and activity. J Leukoc Biol. 2014; 95:337–345.
Article23. Kodama H, Nose M, Niida S, et al. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991; 173:1291–1294.
Article24. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990; 87:4828–4832.
Article25. Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002; 99:111–120.
Article26. Ishida A, Fujita N, Kitazawa R, et al. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J Biol Chem. 2002; 277:26217–26224.
Article27. Wang ZQ, Ovitt C, Grigoriadis AE, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992; 360:741–745.
Article28. Kim HJ, Zhang K, Zhang L, et al. The Src family kinase, Lyn, suppresses osteoclastogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2009; 106:2325–2330.
Article29. Kim HJ, Warren JT, Kim SY, et al. Fyn promotes proliferation, differentiation, survival and function of osteoclast lineage cells. J Cell Biochem. 2010; 111:1107–1113.
Article30. Novack DV, Yin L, Hagen-Stapleton A, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003; 198:771–781.31. Shashidharamurthy R, Machiah D, Aitken JD, et al. Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice. Arthritis Rheum. 2013; 65:1064–1073.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of NFATc1 in Osteoclast Differentiation
- NF-kappaB-Mediated Regulation of Osteoclastogenesis
- Signaling Pathways in Osteoclast Differentiation
- Macrophage colony-stimulating factor promotes the survival of osteoclast precursors by up-regulating Bcl-XL
- Expression of Osteoclastogenesis-related Genes in Rheumatoid Arthritis Synovial Macrophages