Regulation of NFATc1 in Osteoclast Differentiation
- Affiliations
-
- 1Department of Pharmacology, Medical Research Center for Gene Regulation, Chonnam National University Medical School, Gwangju, Korea. nacksung@jnu.ac.kr
- KMID: 2170050
- DOI: http://doi.org/10.11005/jbm.2014.21.4.233
Abstract
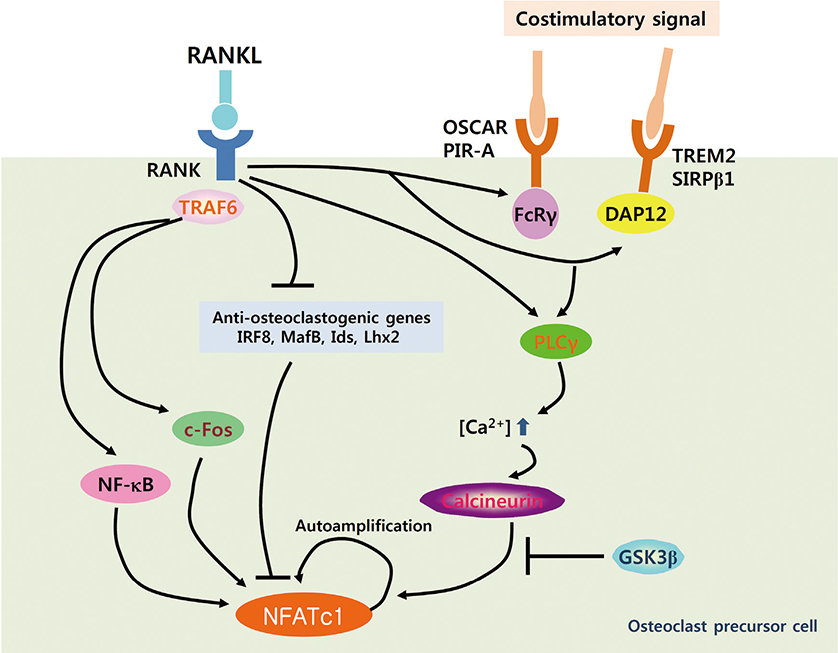
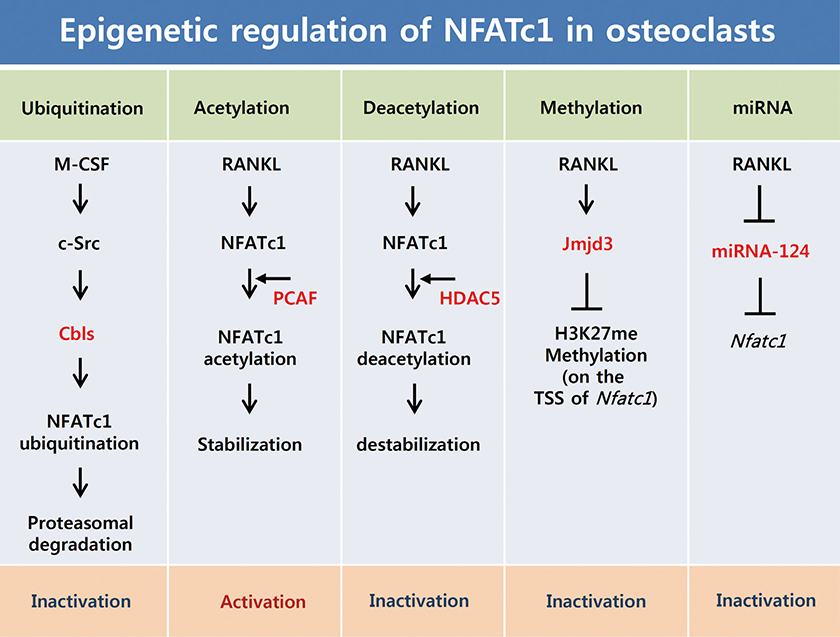
- Osteoclasts are unique cells that degrade the bone matrix. These large multinucleated cells differentiate from the monocyte/macrophage lineage upon stimulation by two essential cytokines, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-kappa B (NF-kappaB) ligand (RANKL). Activation of transcription factors such as microphthalmia transcription factor (MITF), c-Fos, NF-kappaB, and nuclear factor-activated T cells c1 (NFATc1) is required for sufficient osteoclast differentiation. In particular, NFATc1 plays the role of a master transcription regulator of osteoclast differentiation. To date, several mechanisms, including transcription, methylation, ubiquitination, acetylation, and non-coding RNAs, have been shown to regulate expression and activation of NFATc1. In this review, we have summarized the various mechanisms that control NFATc1 regulation during osteoclast differentiation.
MeSH Terms
-
Acetylation
Bone Matrix
Cytokines
Gene Expression Regulation
Macrophage Colony-Stimulating Factor
Methylation
Microphthalmos
NF-kappa B
NFATC Transcription Factors
Osteoclasts*
RANK Ligand
Receptor Activator of Nuclear Factor-kappa B
RNA, Untranslated
T-Lymphocytes
Transcription Factors
Ubiquitin
Ubiquitination
Cytokines
Macrophage Colony-Stimulating Factor
NF-kappa B
NFATC Transcription Factors
RANK Ligand
RNA, Untranslated
Receptor Activator of Nuclear Factor-kappa B
Transcription Factors
Ubiquitin
Figure
Cited by 3 articles
-
Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
Namju Kang, Ki Woo Kim, Dong Min Shin
Korean J Physiol Pharmacol. 2019;23(5):411-417. doi: 10.4196/kjpp.2019.23.5.411.Attenuation of RANKL-induced Osteoclast Formation via p38-mediated NFATc1 Signaling Pathways by Extract of Euphorbia Lathyris L
Ju-Hee Kang, Hyojung Lim, Ji-Eun Jeong, Mijung Yim
J Bone Metab. 2016;23(4):207-214. doi: 10.11005/jbm.2016.23.4.207.Inhibitory Effect of
Rosae Multiflorae Fructus Extracts on the Receptor Activator of NF-κB Ligand-Induced Osteoclastogenesis through Modulation of P38- and Ca2+-Mediated Nuclear Factor of Activated T-Cells Cytoplasmic 1 Expression
Keun Ha Park, Dong Ryun Gu, Min Seuk Kim, Seoung Hoon Lee
J Bone Metab. 2020;27(1):53-63. doi: 10.11005/jbm.2020.27.1.53.
Reference
-
1. Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20:345–357.
Article2. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289:1504–1508.
Article3. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006; 24:33–63.
Article4. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article5. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990; 87:4828–4832.
Article6. Yoshida H, Hayashi S, Kunisada T, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990; 345:442–444.
Article7. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999; 397:315–323.
Article8. Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999; 13:2412–2424.
Article9. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304.
Article10. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.
Article11. Matsumoto M, Kogawa M, Wada S, et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004; 279:45969–45979.
Article12. Kim K, Kim JH, Lee J, et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005; 280:35209–35216.
Article13. Kim Y, Sato K, Asagiri M, et al. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005; 280:32905–32913.
Article14. Winslow MM, Pan M, Starbuck M, et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006; 10:771–782.
Article15. Aliprantis AO, Ueki Y, Sulyanto R, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008; 118:3775–3789.
Article16. Hogan PG, Chen L, Nardone J, et al. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003; 17:2205–2232.
Article17. Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev. 2001; 11:505–512.
Article18. Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002; 109:Suppl. S67–S79.19. Kiani A, Habermann I, Haase M, et al. Expression and regulation of NFAT (nuclear factors of activated T cells) in human CD34+ cells: down-regulation upon myeloid differentiation. J Leukoc Biol. 2004; 76:1057–1065.
Article20. López-Rodríguez C, Aramburu J, Jin L, et al. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001; 15:47–58.21. Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002; 156:771–774.22. Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007; 1116:227–237.
Article23. Hodge MR, Ranger AM, Charles de la Brousse F, et al. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996; 4:397–405.
Article24. Xanthoudakis S, Viola JP, Shaw KT, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996; 272:892–895.
Article25. Yoshida H, Nishina H, Takimoto H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998; 8:115–124.
Article26. Ranger AM, Hodge MR, Gravallese EM, et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998; 8:125–134.
Article27. Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992; 71:577–586.
Article28. Wang ZQ, Ovitt C, Grigoriadis AE, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992; 360:741–745.
Article29. Matsuo K, Galson DL, Zhao C, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004; 279:26475–26480.
Article30. Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–1269.
Article31. Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997; 390:175–179.
Article32. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002; 109:Suppl. S81–S96.33. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004; 18:2195–2224.34. Novack DV, Yin L, Hagen-Stapleton A, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003; 198:771–781.35. Ruocco MG, Maeda S, Park JM, et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005; 201:1677–1687.
Article36. Franzoso G, Carlson L, Xing L, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997; 11:3482–3496.37. Iotsova V, Caamaño J, Loy J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997; 3:1285–1289.38. Takatsuna H, Asagiri M, Kubota T, et al. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J Bone Miner Res. 2005; 20:653–662.
Article39. Koga T, Matsui Y, Asagiri M, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005; 11:880–885.
Article40. Lee J, Kim K, Kim JH, et al. Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood. 2006; 107:2686–2693.
Article41. Kim K, Kim JH, Lee J, et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007; 109:3253–3259.
Article42. Kim JH, Youn BU, Kim K, et al. Lhx2 regulates bone remodeling in mice by modulating RANKL signaling in osteoclasts. Cell Death Differ. 2014; 21:1613–1621.
Article43. Kim K, Lee J, Kim JH, et al. Protein inhibitor of activated STAT 3 modulates osteoclastogenesis by down-regulation of NFATc1 and osteoclast-associated receptor. J Immunol. 2007; 178:5588–5594.
Article44. Zhao B, Takami M, Yamada A, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009; 15:1066–1071.
Article45. Miyauchi Y, Ninomiya K, Miyamoto H, et al. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010; 207:751–762.
Article46. Bird A. Perceptions of epigenetics. Nature. 2007; 447:396–398.
Article47. Yasui T, Hirose J, Aburatani H, et al. Epigenetic regulation of osteoclast differentiation. Ann N Y Acad Sci. 2011; 1240:7–13.
Article48. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003; 33:Suppl. 245–254.
Article49. Yasui T, Hirose J, Tsutsumi S, et al. Epigenetic regulation of osteoclast differentiation: possible involvement of Jmjd3 in the histone demethylation of Nfatc1. J Bone Miner Res. 2011; 26:2665–2671.
Article50. Bae SC, Lee YH. Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene. 2006; 366:58–66.
Article51. Kim JH, Kim K, Jin HM, et al. Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J Biol Chem. 2010; 285:5224–5231.
Article52. Kim JH, Kim K, Youn BU, et al. RANKL induces NFATc1 acetylation and stability via histone acetyltransferases during osteoclast differentiation. Biochem J. 2011; 436:253–262.
Article53. Ambros V. The functions of animal microRNAs. Nature. 2004; 431:350–355.
Article54. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297.55. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007; 8:93–103.
Article56. Kapinas K, Delany AM. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res Ther. 2011; 13:220.
Article57. Lee Y, Kim HJ, Park CK, et al. MicroRNA-124 regulates osteoclast differentiation. Bone. 2013; 56:383–389.
Article58. Mao D, Epple H, Uthgenannt B, et al. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006; 116:2869–2879.
Article59. Beals CR, Sheridan CM, Turck CW, et al. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997; 275:1930–1934.
Article60. Chow CW, Rincón M, Cavanagh J, et al. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997; 278:1638–1641.
Article61. Okamura H, Garcia-Rodriguez C, Martinson H, et al. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004; 24:4184–4195.
Article62. Gómez del Arco P, Martínez-Martínez S, Maldonado JL, et al. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J Biol Chem. 2000; 275:13872–13878.
Article63. Zhu J, Shibasaki F, Price R, et al. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998; 93:851–861.
Article64. Jang HD, Shin JH, Park DR, et al. Inactivation of glycogen synthase kinase-3beta is required for osteoclast differentiation. J Biol Chem. 2011; 286:39043–39050.
Article65. Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004; 428:758–763.
Article66. Kaifu T, Nakahara J, Inui M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003; 111:323–332.
Article67. Mócsai A, Humphrey MB, Van Ziffle JA, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004; 101:6158–6163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tanshinone IIA inhibits osteoclast differentiation through down-regulation of c-Fos and NFATc1
- Poncirin Inhibits Osteoclast Differentiation and Bone Loss through Down-Regulation of NFATc1 In Vitro and In Vivo
- Effects of Liriopis Tuber Water Extract on RANKL-induced Osteoclast Differentiation
- Inhibition of Osteoclast differentiation based on precipitation time of titanium surfaces immersed in modified simulated body fluid
- Inhibitory effect of Chaenomelis Fructus ethanol extract on receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenesis