Korean J Hematol.
2012 Jun;47(2):105-112. 10.5045/kjh.2012.47.2.105.
Arsenic trioxide induces depolymerization of microtubules in an acute promyelocytic leukemia cell line
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. yjmin@uuh.ulsan.kr
- 2Biomedical Research Center, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 3Division of Hematology-Oncology, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2083603
- DOI: http://doi.org/10.5045/kjh.2012.47.2.105
Abstract
- BACKGROUND
Arsenic trioxide (As2O3) is a well-known and effective treatment that can result in clinical remission for patients diagnosed with acute promyelocytic leukemia (APL). The biologic efficacy of As2O3 in APL and solid tumor cells has been explained through its actions on anti-proliferation, anti-angiogenesis, and apoptotic signaling pathways. We theorize that As2O3 activates a pathway that disrupts microtubule dynamics forming abnormal, nonfunctioning mitotic spindles, thus preventing cellular division. In this study, we investigated how As2O3 induces apoptosis by causing microtubule dysfunction.
METHODS
Cultured NB4 cells were treated with As2O3, paclitaxel, and vincristine. Flow cytometric analysis was then performed. An MTT assay was used to determine drug-mediated cytotoxicity. For tubulin polymerization assay, each polymerized or soluble tubulin was measured. Microtubule assembly-disassembly was measured using a tubulin polymerization kit. Cellular microtubules were also observed with fluorescence microscopy.
RESULTS
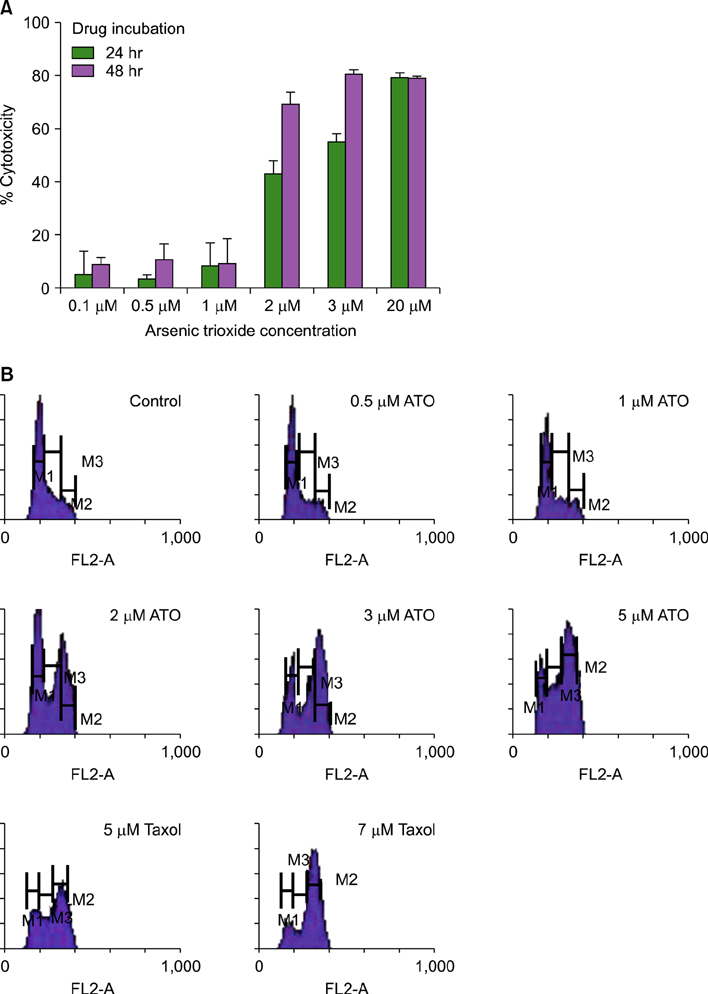
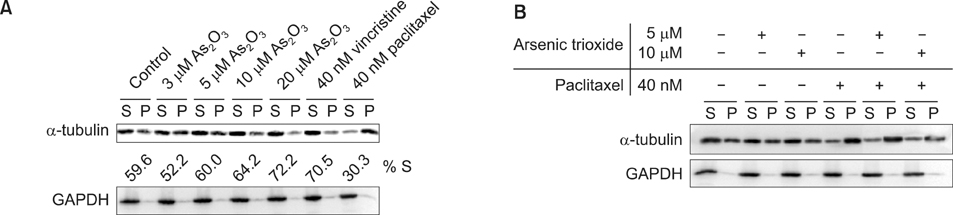
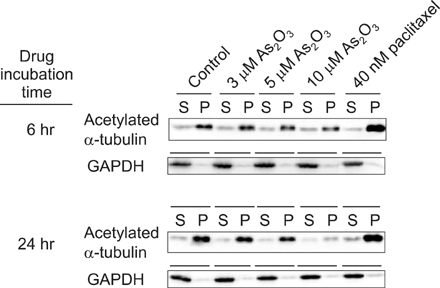
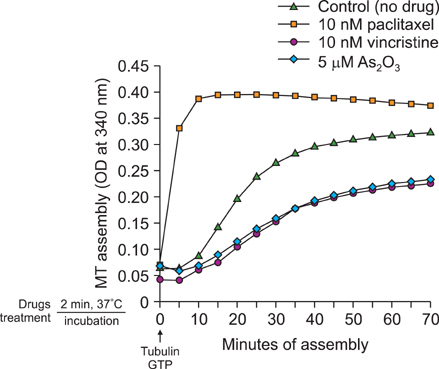
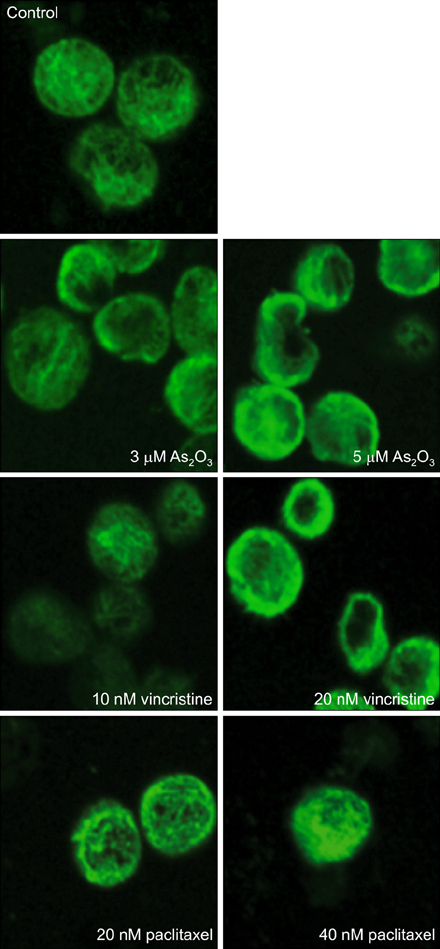
As2O3 treatment disrupted tubulin assembly resulting in dysfunctional microtubules that cause death in APL cells. As2O3 markedly enhanced the amount of depolymerized microtubules. The number of microtubule posttranslational modifications on an individual tubulin decreased with As2O3 concentration. Immunocytochemistry revealed changes in the cellular microtubule network and formation of polymerized microtubules in As2O3-treated cells.
CONCLUSION
The microtubules alterations found with As2O3 treatment suggest that As2O3 increases the depolymerized forms of tubulin in cells and that this is potentially due to arsenite's negative effects on spindle dynamics.
MeSH Terms
-
Antimitotic Agents
Apoptosis
Arsenic
Arsenicals
Cell Line
Fluorescence
Humans
Immunohistochemistry
Leukemia, Promyelocytic, Acute
Microtubules
Oxides
Paclitaxel
Polymerization
Polymers
Protein Processing, Post-Translational
Tubulin
Vincristine
Antimitotic Agents
Arsenic
Arsenicals
Oxides
Paclitaxel
Polymers
Tubulin
Vincristine
Figure
Reference
-
1. Kamimura T, Miyamoto T, Harada M, Akashi K. Advances in therapies for acute promyelocytic leukemia. Cancer Sci. 2011. 102:1929–1937.
Article2. Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002. 62:3893–3903.3. Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007. 18:Suppl 5. v3–v8.
Article4. Barlow SB, Gonzalez-Garay ML, Cabral F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J Cell Sci. 2002. 115:3469–3478.
Article5. Kavallaris M, Tait AS, Walsh BJ, et al. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001. 61:5803–5809.6. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004. 4:253–265.
Article7. Huang SC, Lee TC. Arsenite inhibits mitotic division and perturbs spindle dynamics in HeLa S3 cells. Carcinogenesis. 1998. 19:889–896.
Article8. Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 2002. 62:529–538.
Article9. Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999. 59:776–780.10. Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003. 9:660–664.
Article11. Poruchynsky MS, Kim JH, Nogales E, et al. Tumor cells resistant to a microtubule-depolymerizing hemiasterlin analogue, HTI-286, have mutations in alpha- or beta-tubulin and increased microtubule stability. Biochemistry. 2004. 43:13944–13954.
Article12. Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996. 88:1052–1061.
Article13. Carré M, Carles G, André N, et al. Involvement of microtubules and mitochondria in the antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem Pharmacol. 2002. 63:1831–1842.
Article14. Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003. 421:230.15. Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000. 113:3907–3919.
Article16. Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005. 23:2726–2734.
Article17. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002. 2:48–58.
Article18. Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003. 22:7280–7295.
Article19. Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol Cancer Ther. 2003. 2:597–605.20. Freitas RA, Silva dos, Gimenes Teixeira HL, et al. Apoptosis induction by (+)alpha-tocopheryl succinate in the absence or presence of all-trans retinoic acid and arsenic trioxide in NB4, NB4-R2 and primary APL cells. Leuk Res. 2009. 33:958–963.
Article21. Dong JT, Luo XM. Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res. 1993. 302:97–102.
Article22. Van Wijk R, Welters M, Souren JE, Ovelgonne H, Wiegant FA. Serum-stimulated cell cycle progression and stress protein synthesis in C3H10T1/2 fibroblasts treated with sodium arsenite. J Cell Physiol. 1993. 155:265–272.
Article23. Yih LH, Lee TC. Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res. 2000. 60:6346–6352.24. Minotti AM, Barlow SB, Cabral F. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J Biol Chem. 1991. 266:3987–3994.
Article25. MacRae TH. Tubulin post-translational modifications-enzymes and their mechanisms of action. Eur J Biochem. 1997. 244:265–278.
Article26. Jiang JD, Wang Y, Roboz J, Strauchen J, Holland JF, Bekesi JG. Inhibition of microtubule assembly in tumor cells by 3-bromoacetylamino benzoylurea, a new cancericidal compound. Cancer Res. 1998. 58:2126–2133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Relapsed Acute Promyleocytic Leukemia Induced Remission with Arsenic Trioxide(As2O3)
- Salvage treatments with all-trans retinoic acid and arsenic trioxide-based regimens in acute promyelocytic leukemia
- Remission induction using arsenic trioxide in acute promyelocytic leukemia
- Antitumor Effects of Arsenic Trioxide on Neuroblastoma
- Inducing Apoptosis of NCI-H157 Human Lung Carcinoma Cells via Activation of Caspase Cascade by Combination Treatment with Arsenic Trioxide and Sulindac