Diabetes Metab J.
2011 Oct;35(5):480-488. 10.4093/dmj.2011.35.5.480.
The Effects of Glyburide on Apoptosis and Endoplasmic Reticulum Stress in INS-1 Cells in a Glucolipotoxic Condition
- Affiliations
-
- 1Paik Diabetes Center, Department of Internal Medicine, Inje University College of Medicine, Busan, Korea. pjhdoc@chol.com
- 2Molecular Therapy Lab, Paik Memorial Institute for Clinical Research, Inje University, Busan, Korea.
- 3Department of Internal Medicine, Maryknoll Medical Center, Busan, Korea.
- KMID: 1857503
- DOI: http://doi.org/10.4093/dmj.2011.35.5.480
Abstract
- BACKGROUND
beta-cell death due to endoplasmic reticulum (ER) stress has been regarded as an important pathogenic component of type 2 diabetes. The possibility has been suggested that sulfonylurea, currently being used as one of the main oral hypoglycemic agents of type 2 diabetes, increases ER stress, which could lead to sulfonylurea failure. The authors of the present study examined ER stress of beta-cells in a glucolipotoxic condition using glyburide (GB) in an environment mimicking type 2 diabetes.
METHODS
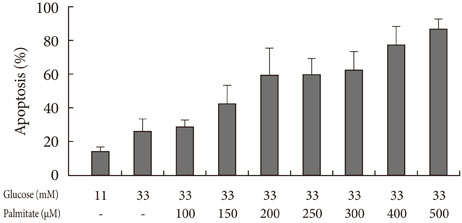
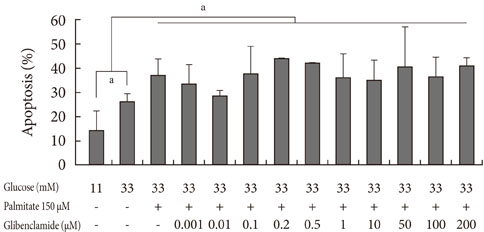
Apoptosis was induced by adding various concentrations of GB (0.001 to 200 microM) to a glucolipotoxic condition using 33 mM glucose, and the effects of varied concentrations of palmitate were evaluated via annexin V staining. The markers of ER stress and pro-apoptotic markers were assessed by Western blotting and semi-quantitative reverse transcription-polymerase chain reaction. Additionally, the anti-apoptotic markers were evaluated.
RESULTS
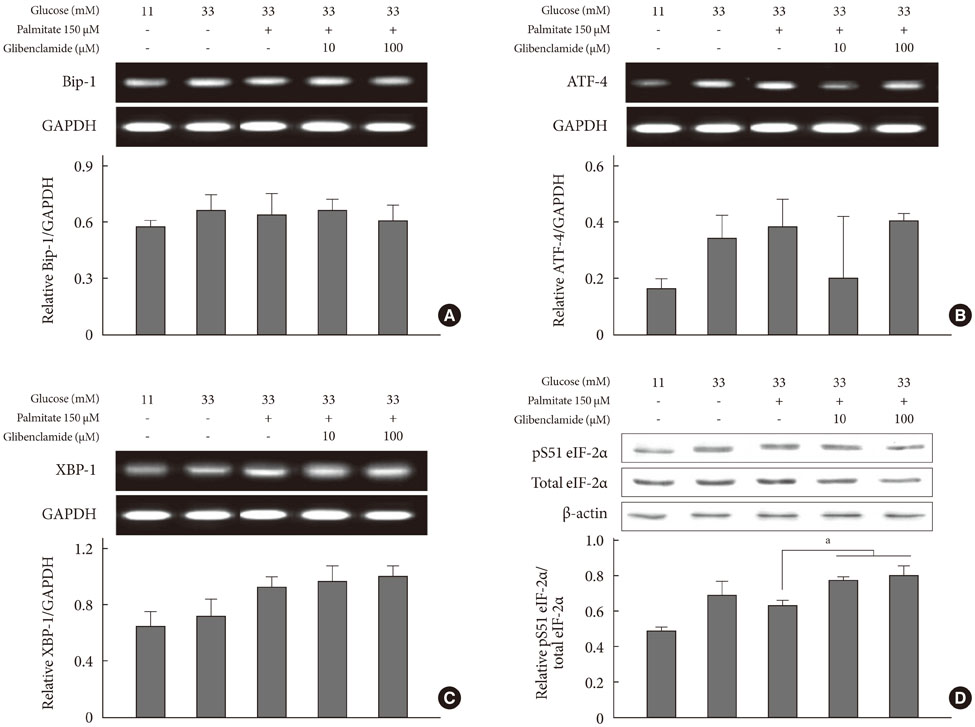
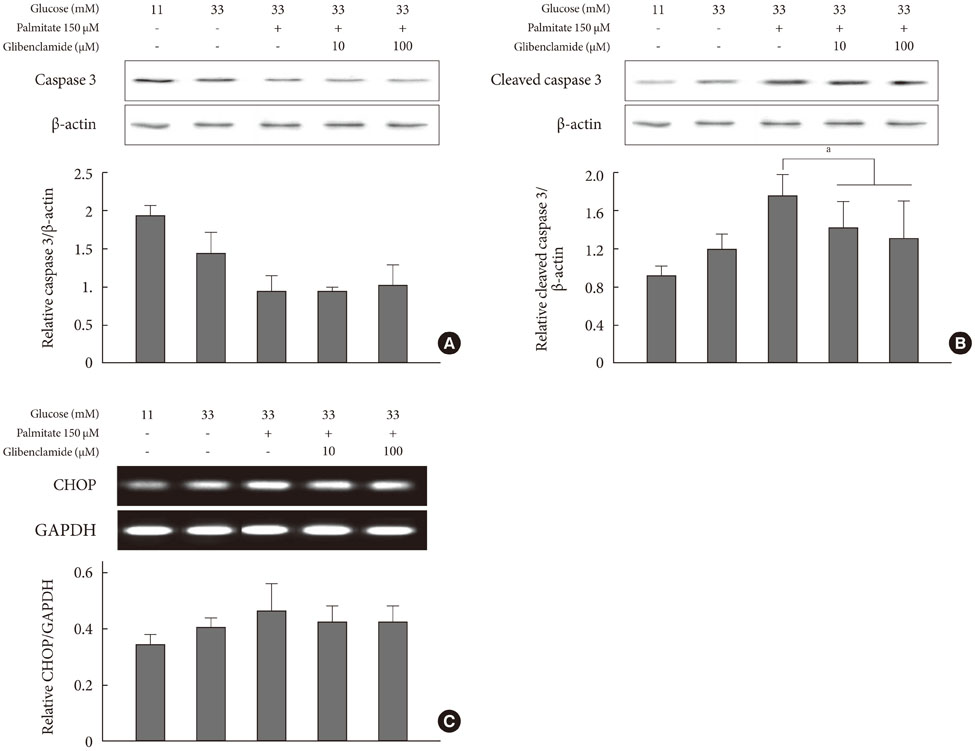
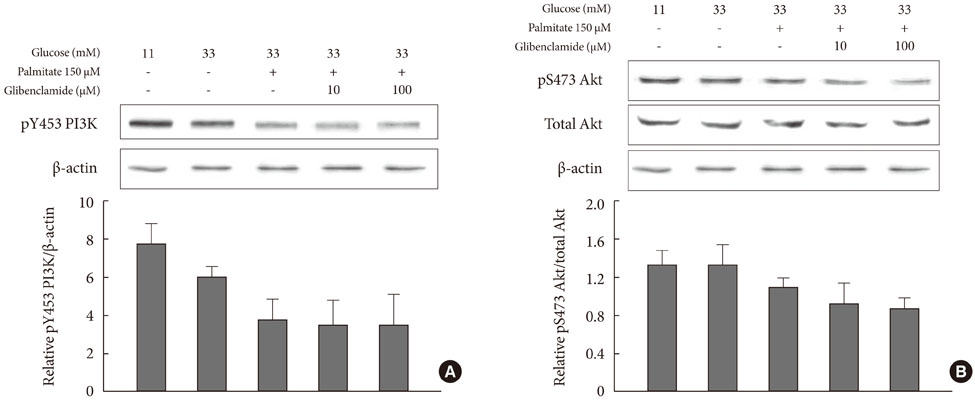
Addition of any concentration of GB in 150 microM palmitate and 33 mM glucose did not increase apoptosis. The expression of phosphorylated eukaryotic initiation factor (eIF-2alpha) was increased and cleaved caspase 3 was decreased by adding GB to a glucolipotoxic condition. However, other ER stress-associated markers such as Bip-1, X-box binding protein-1, ATF-4 and C/EBP-homologous protein transcription factor and anti-apoptotic markers phosphor-p85 phosphatidylinositol 3-kinase and phosphorylation of Akt did not change significantly.
CONCLUSION
GB did not show further deleterious effects on the degree of apoptosis or ER stress of INS-1 cells in a glucolipotoxic condition. Increased phosphorylation of eIF-2alpha may attenuate ER stress for adaptation to increased ER protein load.
MeSH Terms
-
Annexin A5
Apoptosis
Blotting, Western
Caspase 3
Endoplasmic Reticulum
Endoplasmic Reticulum Stress
Eukaryotic Initiation Factor-2
Glucose
Glyburide
Hypoglycemic Agents
Insulin-Secreting Cells
Peptide Initiation Factors
Phosphatidylinositol 3-Kinase
Phosphorylation
Transcription Factors
Annexin A5
Caspase 3
Eukaryotic Initiation Factor-2
Glucose
Glyburide
Hypoglycemic Agents
Peptide Initiation Factors
Phosphatidylinositol 3-Kinase
Transcription Factors
Figure
Reference
-
1. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003. 52:102–110.2. Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008. 9:329–343.3. Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002. 143:339–342.4. LeRoith D, Taylor SI, Olefsky JM. Processing of the insulin molecule. Diabetes mellitus. 2004. Philadelphia: Lippincott Williams & Wilkins;27–50.5. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007. 8:519–529.6. Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007. 32:469–476.7. Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008. 29:42–61.8. Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002. 3:411–421.9. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000. 2:326–332.10. Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, Benzi L, Del Prato S, Marchetti P. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications. 2005. 19:60–64.11. Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells: a process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998. 273:33501–33507.12. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006. 355:2427–2443.13. Takahashi A, Nagashima K, Hamasaki A, Kuwamura N, Kawasaki Y, Ikeda H, Yamada Y, Inagaki N, Seino Y. Sulfonylurea and glinide reduce insulin content, functional expression of K(ATP) channels, and accelerate apoptotic beta-cell death in the chronic phase. Diabetes Res Clin Pract. 2007. 77:343–350.14. Qian L, Zhang S, Xu L, Peng Y. Endoplasmic reticulum stress in beta cells: latent mechanism of secondary sulfonylurea failure in type 2 diabetes? Med Hypotheses. 2008. 71:889–891.15. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005. 90:501–506.16. Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001. 50:69–76.17. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008. 29:351–366.18. Elks ML. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993. 133:208–214.19. Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995. 80:1584–1590.20. Ritz-Laser B, Meda P, Constant I, Klages N, Charollais A, Morales A, Magnan C, Ktorza A, Philippe J. Glucose-induced preproinsulin gene expression is inhibited by the free fatty acid palmitate. Endocrinology. 1999. 140:4005–4014.21. Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001. 50:1771–1777.22. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998. 95:2498–2502.23. El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003. 144:4154–4163.24. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007. 50:752–763.25. Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007. 56:2016–2027.26. Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007. 50:2486–2494.27. Inoguchi T, Umeda F, Kakimoto M, Sako Y, Ishii H, Noda K, Kunisaki M, Imamura M, Yu HY, Etoh T, Yoshikawa H, Aoki T, Hashimoto T, Nawata H. Chronic sulfonylurea treatment and hyperglycemia aggravate disproportionately elevated plasma proinsulin levels in patients with type 2 diabetes. Endocr J. 2000. 47:763–770.28. Dworacka M, Abramczyk M, Winiarska H, Kuczynski S, Borowska M, Szczawinska K. Disproportionately elevated proinsulin levels in type 2 diabetic patients treated with sulfonylurea. Int J Clin Pharmacol Ther. 2006. 44:14–21.29. Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 2010. 17:107–112.30. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001. 7:1153–1163.31. Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006. 4:491–497.32. Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005. 11:757–764.33. Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001. 7:1165–1176.34. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006. 4:245–254.35. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.36. Sawada F, Inoguchi T, Tsubouchi H, Sasaki S, Fujii M, Maeda Y, Morinaga H, Nomura M, Kobayashi K, Takayanagi R. Differential effect of sulfonylureas on production of reactive oxygen species and apoptosis in cultured pancreatic beta-cell line, MIN6. Metabolism. 2008. 57:1038–1045.37. Ishigaki S, Fonseca SG, Oslowski CM, Jurczyk A, Shearstone JR, Zhu LJ, Permutt MA, Greiner DL, Bortell R, Urano F. AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1. Cell Death Differ. 2010. 17:774–786.38. Price J, Zaidi AK, Bohensky J, Srinivas V, Shapiro IM, Ali H. Akt-1 mediates survival of chondrocytes from endoplasmic reticulum-induced stress. J Cell Physiol. 2010. 222:502–508.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low glibenclamide concentrations affect endoplasmic reticulum stress in INS-1 cells under glucotoxic or glucolipotoxic conditions
- Endoplasmic Reticulum Stress Responses and Apoptosis
- The Effects of Exendin-4 on IRS-2 Expression and Phosphorylation in INS-1 Cells
- Endoplasmic Reticulum Stress and Diabetes
- The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells