Korean Circ J.
2010 Oct;40(10):520-526. 10.4070/kcj.2010.40.10.520.
Alagebrium Chloride, a Novel Advanced Glycation End-Product Cross Linkage Breaker, Inhibits Neointimal Proliferation in a Diabetic Rat Carotid Balloon Injury Model
- Affiliations
-
- 1Cardiology Division, Yonsei University College of Medicine, Seoul, Korea. shpark0530@yuhs.ac
- 2Yonsei Cardiovascular Center and Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Endocrinology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1826141
- DOI: http://doi.org/10.4070/kcj.2010.40.10.520
Abstract
- BACKGROUND AND OBJECTIVES
Vascular perturbation induced by advanced glycation end-products (AGEs) leads to progression of atherosclerosis, plaque instability, and vascular inflammation, which results in a higher risk of neointimal proliferation. Here we investigated the inhibitory effect of alagebrium chloride (ALT-711), a breaker of AGE-based cross links, on neointimal proliferation in a carotid artery balloon injury model in diabetic rats induced by streptozotocin (STZ).
MATERIALS AND METHODS
Rat aortic vascular smooth muscle cells (RASMCs) were treated with 1-100 microM of alagebrium added 24 hours before the addition of AGEs. This in vivo study was done using 8-week-old male rats that were injected intraperitoneally with 80 mg/kg STZ. Sixteen weeks later, the diabetic rats were treated with 10 mg/kg alagebrium for 4 weeks, after which carotid artery balloon injury was induced. After 4 weeks, the animals were sacrificed for histological analysis.
RESULTS
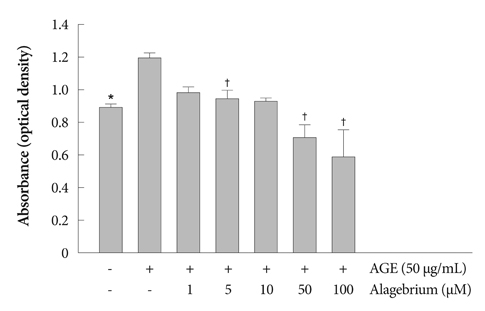
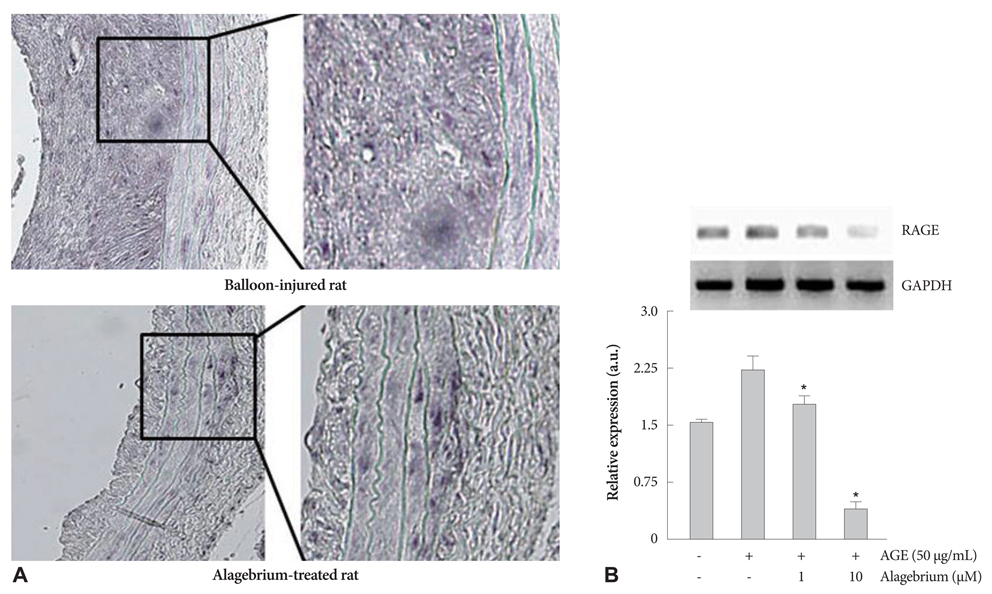
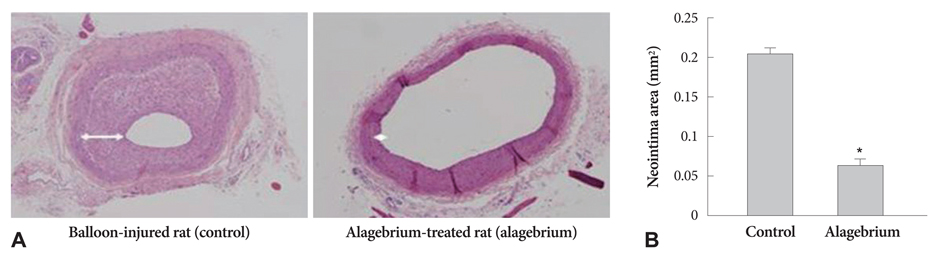
Proliferation of RASMCs was significantly inhibited in alagebrium-treated cells. Alagebrium dose-dependently inhibited AGE-mediated formation of reactive oxygen species (ROS), extracellular signal-regulated kinase phosphorylation, and cyclooxygenase-2 expression. The cellular mechanisms of AGE-induced connective tissue and extracellular matrix expression were decreased in the alagebrium-treated group. This in vivo study shows that expression of AGE receptors and neointima hyperplasia are significantly suppressed in balloon-injured rats treated with alagebrium.
CONCLUSION
Alagebrium treatment in diabetic rats significantly inhibits neointimal hyperplasia after carotid balloon injury due to its inhibition of intracellular ROS synthesis, which results in inhibition of RASMCs proliferation.
MeSH Terms
-
Animals
Atherosclerosis
Carotid Arteries
Connective Tissue
Cyclooxygenase 2
Extracellular Matrix
Humans
Hyperplasia
Inflammation
Male
Muscle, Smooth, Vascular
Neointima
Phosphorylation
Phosphotransferases
Rats
Reactive Oxygen Species
Streptozocin
Thiazoles
Cyclooxygenase 2
Phosphotransferases
Reactive Oxygen Species
Streptozocin
Thiazoles
Figure
Reference
-
1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. Circulation. 1979. 59:8–13.2. Ha JK, Han DC, Hwang KW, et al. Metabolic syndrome and risk of in-stent restenosis: clinical outcomes in patients undergoing percutaneous coronary intervention. Korean Circ J. 2007. 37:567–573.3. Park JS, Seok JH, Hong GR, Shin DG, Kim YJ, Shim BS. Types of in-stent restenosis and predictive factors for diffuse type in-stent restenosis. Korean Circ J. 2001. 31:1135–1141.4. Nielsen TT, Bøtker HE. Percutaneous coronary intervention in diabetic patients: a problem? Horm Metab Res. 2005. 37:Suppl 1. 83–89.5. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994. 331:489–495.6. Vaitkevicius PV, Lane M, Spurgeon H, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A. 2001. 98:1171–1175.7. Candido R, Forbes JM, Thomas MC, et al. A breaker of advanced glycation end products attenuates diabetes induced myocardial structural changes. Circ Res. 2003. 92:785–792.8. Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX, Degroof RC. The effect of alagebrium chloride(ALT-711), a novel glucose cross link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005. 11:191–195.9. Cooper ME. Impotance of advanced glycation end products in diabetes associated cardiovascular and renal disease. Am J Hypertens. 2004. 17:31S–38S.10. Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003. 93:1159–1169.11. Kass DA, Shapiro EP, Kaawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end product crosslink breakers. Circulation. 2001. 104:1464–1470.12. Wolffenbuttel BH, Boulanger CM, Crijns FR, et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A. 1998. 95:4630–4634.13. Ettenson DS, Gotlieb AI. Centrosomes, microtubules, and microfilaments in the reendothelialization and remodeling of double-sided in vitro wound. Lab Invest. 1992. 66:722–733.14. Villa AE, Guzman LA, Chen W, Golomb G, Levy RJ, Topol EJ. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest. 1994. 93:1243–1249.15. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury: I. smooth muscle growth in the absence of endothelium. Lab Invest. 1983. 49:327–333.16. Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003. 111:959–972.17. Naka Y, Bucciarelli LG, Wendt T, et al. RAGE axis: animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004. 24:1342–1349.18. Shim CY, Park S, Yoon SJ, et al. Association of RAGE gene polymorphisms with in-stent restenosis in non-diabetic Korean population. Cardiology. 2007. 107:261–268.19. Susic D, Varagic J, Ahn J, Frohlich ED. Crosslink breakers: a new approach to cardiovascular therapy. Curr Opin Cardiol. 2004. 19:336–340.20. Forbes JM, Yee LT, Thallas V, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004. 53:1813–1823.21. Zhou Z, Wang K, Penn MS, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003. 107:2238–2243.22. Yue TL, Bao W, Gu JL, et al. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005. 54:554–562.23. Park S, Lim S, Chang W, et al. The inhibition of insulin-stimulated proliferation of vascular smooth muscle cells by rosiglitazone is mediated by the Akt-mTOR-P70S6K pathway. Yonsei Med J. 2008. 49:592–600.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Breakdown of Preformed Peritoneal Advanced Glycation End Products by Intraperitoneal Alagebrium
- Effect of Udenafil on Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Rat Carotid Artery Injury Model
- The Effect of External Beam Radiation on Neointimal Formation in the Rat Carotid Injury Model
- The Role of Advanced Glycation End Products in Diabetic Vascular Complications
- The neointimal hyperplasia effect of erythropoietin on carotid artery injury model of rat