Tuberc Respir Dis.
2006 Apr;60(4):451-463. 10.4046/trd.2006.60.4.451.
The Role of Poly(ADP-ribose) Polymerase-1 in Ventilator-Induced Lung Injury
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Korea University, Seoul, Korea. kkhchest@korea.ac.kr
- 2Institute of Human Genomic Study, Ansan Hospital, Korea University Medical Center, Ansan, Korea.
- KMID: 1719370
- DOI: http://doi.org/10.4046/trd.2006.60.4.451
Abstract
-
BACKGROUND: Reactive oxygen species (ROS) take center stage as executers in ventilator-induced lung injury (VILI). The protein with DNA-damage scanning activity, poly (ADP-ribose) polymerase-1 (PARP1), signals DNA rupture and participates in base-excision repair. Paradoxically,overactivation of PARP1 in response to massive genotoxic injury such as ROS can induce cell death through beta-nicotinamide adenine dinucleotide (NAD+) depletion, resulting in inflammation. The purpose of this study is to investigate the role of PARP1 and the effect of its inhibitor in VILI.
METHODS
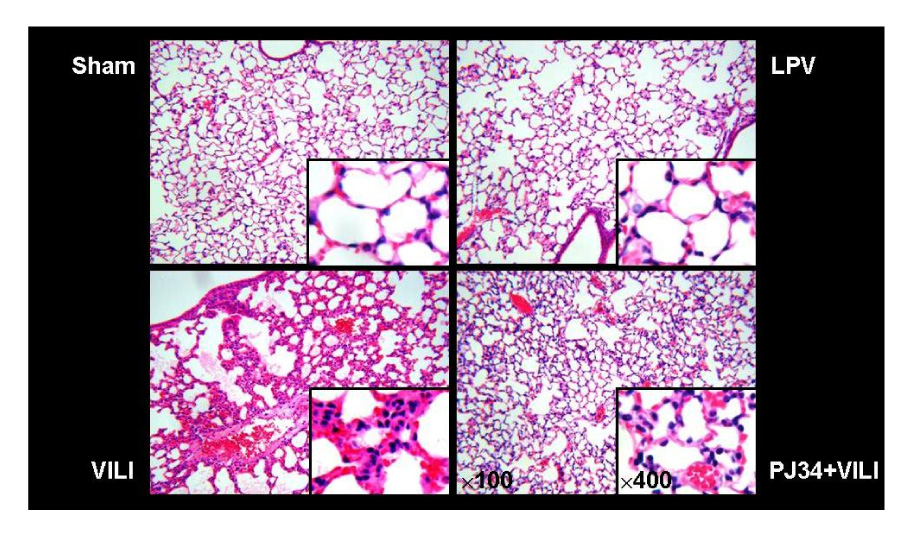
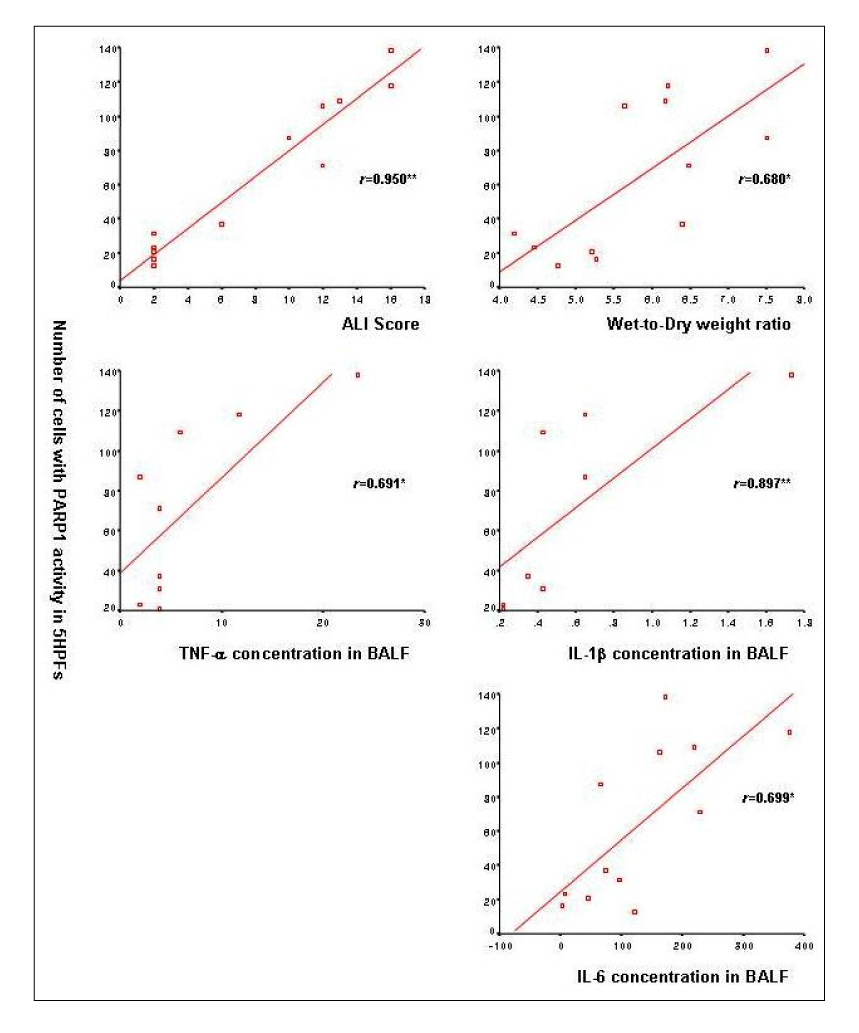
Forty-eight male C57BL/6 mice were divided into sham, lung protective ventilation(LPV), VILI, and PARP1 inhibitor (PJ34)+VILI (PJ34+VILI) groups. Mechanical ventilator setting for the LPV group was PIP 15 cmH2O + PEEP 3 cmH2O + RR 90/min + 2 hours. The VILI and PJ34+VILI groups were ventilated on a setting of PIP 40 cmH2O + PEEP 0 cmH2O + RR 90/min + 2 hours. As a PARP1 inhibitor for the PJ34+VILI group, 20 mg/Kg of PJ34 was treated intraperitoneally 2 hours before mechanical ventilation. Wet-to-dry weight ratio and acute lung injury (ALI) score were measured to determine the degree of VILI. PARP1 activity was evaluated by using an immunohistochemical method utilizing biotinylated NAD. Myeloperoxidase (MPO) activity and the concentration of inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6 were measured in bronchoalveolar lavage fluid (BALF).
RESULTS
In the PJ34+VILI group, PJ34 pretreatment significantly reduced the degree of lung injury, compared with the VILI group (p<0.05). The number of cells expressing PARP1 activity was significantly increased in the VILI group, but significantly decreased in the PJ34+VILI group (p=0.001). In BALF, MPO activity, TNF-alpha, IL-1beta, and IL-6 were also significantly lower in the PJ34+VILI group (all, p<0.05).
CONCLUSION
PARP1 overactivation plays a major role in the mechanism of VILI. PARP1 inhibitor prevents VILI, and decreases MPO activity and inflammatory cytokines.
Keyword
MeSH Terms
-
Acute Lung Injury
Adenine
Animals
Bronchoalveolar Lavage Fluid
Cell Death
Cytokines
DNA
Humans
Inflammation
Interleukin-6
Interleukins
Lung
Lung Injury
Male
Mice
NAD
Peroxidase
Poly Adenosine Diphosphate Ribose*
Reactive Oxygen Species
Respiration, Artificial
Rupture
Tumor Necrosis Factor-alpha
Ventilator-Induced Lung Injury*
Ventilators, Mechanical
Adenine
Cytokines
DNA
Interleukin-6
Interleukins
NAD
Peroxidase
Poly Adenosine Diphosphate Ribose
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. International consensus conferences in intensive care medicine: ventilator-associated Lung Injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societe de Reanimation de Langue Francaise, and was approved by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999. 160:2118–2124.2. Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998. 157:1721–1725.3. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. 342:1301–1308.4. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998. 338:347–354.5. Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998. 110:482–488.6. Uhlig S, Uhlig U. Pharmacological interventions in ventilator-induced lung injury. Trends Pharmacol Sci. 2004. 25:592–600.7. Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004. 26:882–893.8. Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003. 31:446–454.9. Huber A, Bai P, de Murcia JM, de Murcia G. PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair. 2004. 3:1103–1108.10. Nguewa PA, Fuertes MA, Valladares B, Alonso C, Perez JM. Poly(ADP-ribose) polymerases: homology, structural domains and functions: novel therapeutical applications. Prog Biophys Mol Biol. 2005. 88:143–172.11. Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002. 54:375–429.12. D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999. 342:249–268.13. Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001. 477:97–110.14. Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985. 101:4–15.15. Berger NA, Berger SJ. Metabolic consequences of DNA damage: the role of poly (ADP-ribose) polymerase as mediator of the suicide response. Basic Life Sci. 1986. 38:357–363.16. Berger NA, Whitacre CM, Hashimoto H, Berger SJ, Chatterjee S. NAD and poly(ADP-ribose) regulation of proteins involved in response to cellular stress and DNA damage. Biochimie. 1995. 77:364–367.17. Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997. 3:1089–1095.18. Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999. 96:13978–13982.19. Heller B, Wang ZQ, Wagner EF, Radons J, Burkle A, Fehsel K, et al. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995. 270:11176–11180.20. Szabo C, Zingarelli B, O'Connor M, Salzman AL. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996. 93:1753–1758.21. Virag L, Salzman AL, Szabo C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J Immunol. 1998. 161:3753–3759.22. Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994. 263:687–689.23. Zingarelli B, O'Connor M, Wong H, Salzman AL, Szabo C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996. 156:350–358.24. Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998. 83:85–94.25. Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998. 157:294–323.26. Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg. 2001. 92:428–436.27. Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001. 7:108–113.28. Zhang J. Use of biotinylated NAD to label and purify ADP-ribosylated proteins. Methods Enzymol. 1997. 280:255–265.29. Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, et al. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002. 165:372–377.30. Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996. 270:L836–L845.31. Tsuno K, Miura K, Takeya M, Kolobow T, Morioka T. Histopathologic pulmonary changes from mechanical ventilation at high peak airway pressures. Am Rev Respir Dis. 1991. 143:1115–1120.32. Woo SW, Hedley-Whyte J. Macrophage accumulation and pulmonary edema due to thoracotomy and lung over inflation. J Appl Physiol. 1972. 33:14–21.33. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997. 99:944–952.34. Matsuoka T, Kawano T, Miyasaka K. Role of high-frequency ventilation in surfactant-depleted lung injury as measured by granulocytes. J Appl Physiol. 1994. 76:539–544.35. Sugiura M, McCulloch PR, Wren S, Dawson RH, Froese AB. Ventilator pattern influences neutrophil influx and activation in atelectasis-prone rabbit lung. J Appl Physiol. 1994. 77:1355–1365.36. Takata M, Abe J, Tanaka H, Kitano Y, Doi S, Kohsaka T, et al. Intraalveolar expression of tumor necrosis factor-alpha gene during conventional and high-frequency ventilation. Am J Respir Crit Care Med. 1997. 156:272–279.37. Okuyama K. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am J Respir Crit Care Med. 1994. 150:1550–1554.38. Zhang H, Downey GP, Suter PM, Slutsky AS, Ranieri VM. Conventional mechanical ventilation is associated with bronchoalveolar lavage-induced activation of polymorphonuclear leukocytes: a possible mechanism to explain the systemic consequences of ventilator-induced lung injury in patients with ARDS. Anesthesiology. 2002. 97:1426–1433.39. Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004. 9:43–53.40. Li LF, Yu L, Quinn DA. Ventilation-induced neutrophil infiltration depends on c-Jun N-terminal kinase. Am J Respir Crit Care Med. 2004. 169:518–524.41. Rimensberger PC, Fedorko L, Cutz E, Bohn DJ. Attenuation of ventilator-induced acute lung injury in an animal model by inhibition of neutrophil adhesion by leumedins (NPC 15669). Crit Care Med. 1998. 26:548–555.42. Ohta N, Shimaoka M, Imanaka H, Nishimura M, Taenaka N, Kiyono H, et al. Glucocorticoid suppresses neutrophil activation in ventilator-induced lung injury. Crit Care Med. 2001. 29:1012–1016.43. Chavolla-Calderon M, Bayer MK, Fontan JJ. Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation. J Clin Invest. 2003. 111:973–980.44. Althaus FR, Kleczkowska HE, Malanga M, Muntener CR, Pleschke JM, Ebner M, et al. Poly ADP-ribosylation: a DNA break signal mechanism. Mol Cell Biochem. 1999. 193:5–11.45. Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis: caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999. 274:22932–22940.46. Boulares AH, Zoltoski AJ, Yakovlev A, Xu M, Smulson ME. Roles of DNA fragmentation factor and poly(ADP-ribose) polymerase in an amplification phase of tumor necrosis factor-induced apoptosis. J Biol Chem. 2001. 276:38185–38192.47. Ding R, Pommier Y, Kang VH, Smulson M. Depletion of poly(ADP-ribose) polymerase by antisense RNA expression results in a delay in DNA strand break rejoining. J Biol Chem. 1992. 267:12804–12812.48. Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005. 4:421–440.49. Szabo C, Lim LH, Cuzzocrea S, Getting SJ, Zingarelli B, Flower RJ, et al. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997. 186:1041–1049.50. Zingarelli B, Hake PW, O'Connor M, Denenberg A, Wong HR, Kong S, et al. Differential regulation of activator protein-1 and heat shock factor-1 in myocardial ischemia and reperfusion injury: role of poly (ADP-ribose) polymerase-1. Am J Physiol Heart Circ Physiol. 2004. 286:H1408–H1415.51. Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L834–L841.52. Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, et al. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001. 7:255–260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells
- Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation
- The Important Role of Poly ADP-Ribose Polymerase Inhibitor in Prostate Cancer
- Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells
- Increased Poly (ADP-ribose) Polymerase Activation in the 6-Hydroxydopamine Induced Dopaminergic Neuronal Cell Death