Anat Cell Biol.
2016 Jun;49(2):79-87. 10.5115/acb.2016.49.2.79.
Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells
- Affiliations
-
- 1Department of Biomedicine and Drug Development, Jeju National University, Jeju, Korea. jinu.kim@jejunu.ac.kr
- 2Department of Anatomy, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2308914
- DOI: http://doi.org/10.5115/acb.2016.49.2.79
Abstract
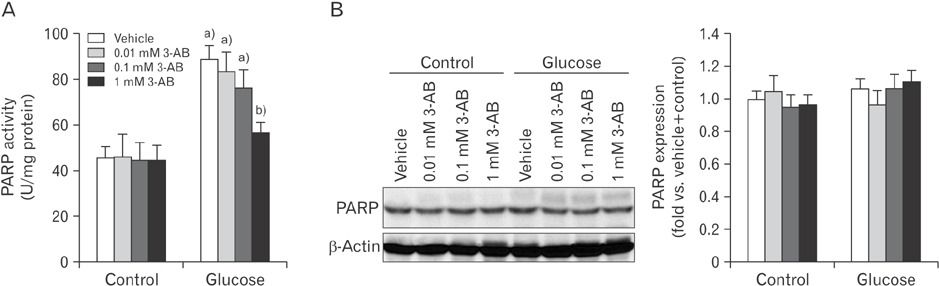
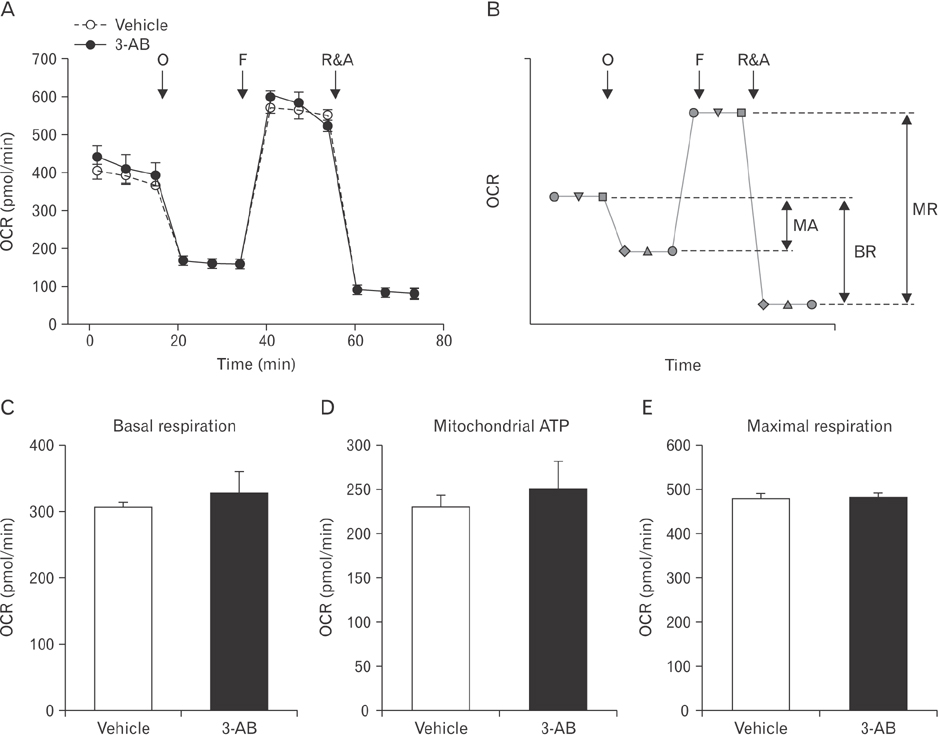
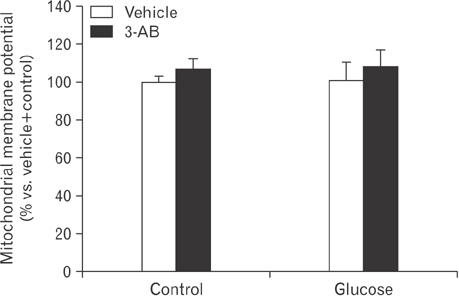
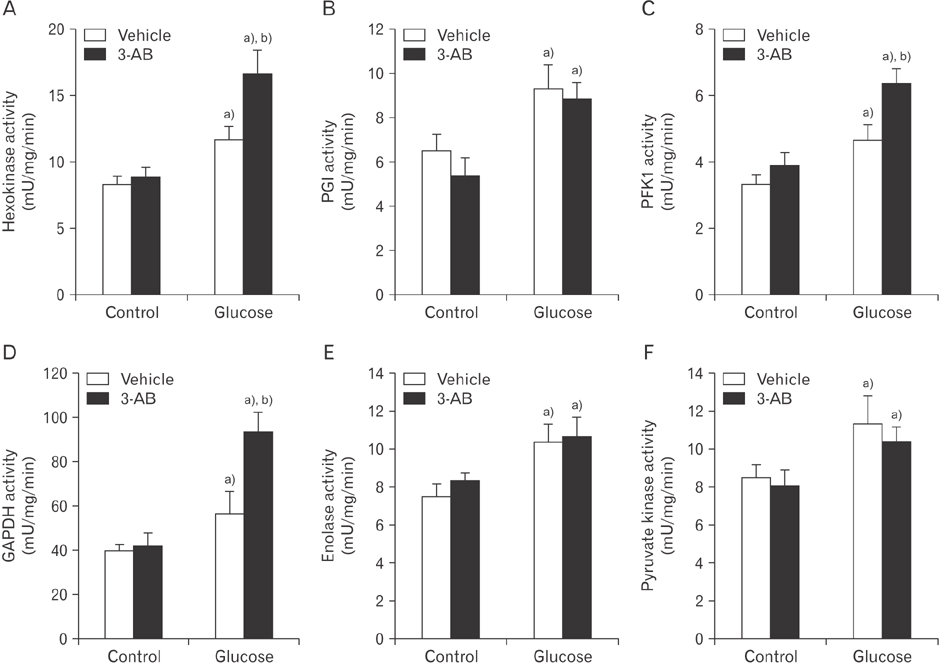
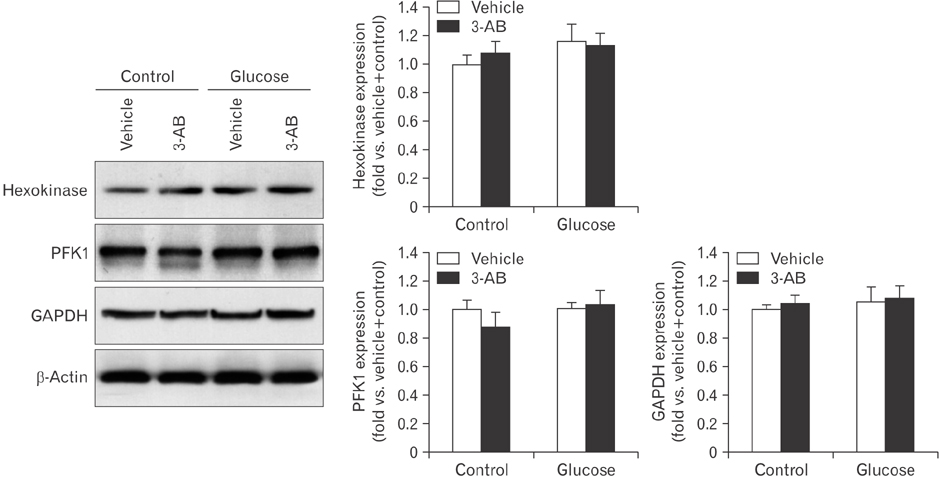
- After renal injury, selective damage occurs in the proximal tubules as a result of inhibition of glycolysis. The molecular mechanism of damage is not known. Poly(ADP-ribose) polymerase (PARP) activation plays a critical role of proximal tubular cell death in several renal disorders. Here, we studied the role of PARP on glycolytic flux in pig kidney proximal tubule epithelial LLC-PK1 cells using XFp extracellular flux analysis. Poly(ADP-ribosyl)ation by PARP activation was increased approximately 2-fold by incubation of the cells in 10 mM glucose for 30 minutes, but treatment with the PARP inhibitor 3-aminobenzamide (3-AB) does-dependently prevented the glucose-induced PARP activation (approximately 14.4% decrease in 0.1 mM 3-AB-treated group and 36.7% decrease in 1 mM 3-AB-treated group). Treatment with 1 mM 3-AB significantly enhanced the glucose-mediated increase in the extracellular acidification rate (61.1±4.3 mpH/min vs. 126.8±6.2 mpH/min or approximately 2-fold) compared with treatment with vehicle, indicating that PARP inhibition increases only glycolytic activity during glycolytic flux including basal glycolysis, glycolytic activity, and glycolytic capacity in kidney proximal tubule epithelial cells. Glucose increased the activities of glycolytic enzymes including hexokinase, phosphoglucose isomerase, phosphofructokinase-1, glyceraldehyde-3-phosphate dehydrogenase, enolase, and pyruvate kinase in LLC-PK1 cells. Furthermore, PARP inhibition selectively augmented the activities of hexokinase (approximately 1.4-fold over vehicle group), phosphofructokinase-1 (approximately 1.6-fold over vehicle group), and glyceraldehyde-3-phosphate dehydrogenase (approximately 2.2-fold over vehicle group). In conclusion, these data suggest that PARP activation may regulate glycolytic activity via poly(ADP-ribosyl)ation of hexokinase, phosphofructokinase-1, and glyceraldehyde-3-phosphate dehydrogenase in kidney proximal tubule epithelial cells.
Keyword
MeSH Terms
-
Animals
Cell Death
Epithelial Cells*
Glucose
Glucose-6-Phosphate Isomerase
Glycolysis
Hexokinase
Kidney*
LLC-PK1 Cells
Oxidoreductases
Phosphofructokinase-1
Phosphopyruvate Hydratase
Poly Adenosine Diphosphate Ribose*
Poly(ADP-ribose) Polymerases*
Pyruvate Kinase
Swine
Glucose
Glucose-6-Phosphate Isomerase
Hexokinase
Oxidoreductases
Phosphofructokinase-1
Phosphopyruvate Hydratase
Poly Adenosine Diphosphate Ribose
Poly(ADP-ribose) Polymerases
Pyruvate Kinase
Figure
Cited by 2 articles
-
Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity
Sang Pil Yoon, Jinu Kim
Anat Cell Biol. 2018;51(3):189-199. doi: 10.5115/acb.2018.51.3.189.Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
Daeun Moon, Jinu Kim
Anat Cell Biol. 2019;52(3):312-323. doi: 10.5115/acb.18.192.
Reference
-
1. Kraus WL, Lis JT. PARP goes transcription. Cell. 2003; 113:677–683.2. Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999; 96:13978–13982.3. Berger NA, Berger SJ. Metabolic consequences of DNA damage: the role of poly (ADP-ribose) polymerase as mediator of the suicide response. Basic Life Sci. 1986; 38:357–363.4. Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R1834–R1840.5. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci. 2015; 65:105–111.6. Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.7. Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015; 48:66–74.8. Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011; 301:F450–F459.9. Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998; 275(5 Pt 2):F623–F631.10. Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol. 1985; 248(4 Pt 2):F522–F526.11. Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol. 1981; 240:F492–F500.12. Chesney J. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care. 2006; 9:535–539.13. Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011; 76:211–216.14. Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2005; 45:269–290.15. Gagne JP, Isabelle M, Lo KS, Bourassa S, Hendzel MJ, Dawson VL, Dawson TM, Poirier GG. Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res. 2008; 36:6959–6976.16. Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly (ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003; 112:1049–1057.17. Kim J, Devalaraja-Narashimha K, Padanilam BJ. TIGAR regulates glycolysis in ischemic kidney proximal tubules. Am J Physiol Renal Physiol. 2015; 308:F298–F308.18. Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim. 2015; 51:72–78.19. Althaus FR, Richter C. ADP-ribosylation of proteins: enzymology and biological significance. Mol Biol Biochem Biophys. 1987; 37:1–237.20. Krietsch J, Rouleau M, Pic É, Ethier C, Dawson TM, Dawson VL, Masson JY, Poirier GG, Gagné JP. Reprogramming cellular events by poly(ADP-ribose)-binding proteins. Mol Aspects Med. 2013; 34:1066–1087.21. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011; 27:441–464.22. Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagné JP, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A. 2014; 111:10209–10214.23. Weber G. Enzymology of cancer cells (second of two parts). N Engl J Med. 1977; 296:541–551.24. Mohr S, Stamler JS, Brüne B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994; 348:223–227.25. Devalaraja-Narashimha K, Padanilam BJ. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol. 2009; 20:95–103.26. Zwerschke W, Mazurek S, Stöckl P, Hütter E, Eigenbrodt E, Jansen-Dürr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J. 2003; 376(Pt 2):403–411.27. Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994; 269:31484–31490.28. Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011; 13:461–468.29. Pang J, Gong H, Xi C, Fan W, Dai Y, Zhang TM. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J Cell Biochem. 2011; 112:299–306.30. Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013; 4:e532.31. Dhanya R, Arun KB, Syama HP, Nisha P, Sundaresan A, Santhosh Kumar TR, Jayamurthy P. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014; 158:546–554.32. Petrescu AD, McIntosh AL, Storey SM, Huang H, Martin GG, Landrock D, Kier AB, Schroeder F. High glucose potentiates L-FABP mediated fibrate induction of PPARalpha in mouse hepatocytes. Biochim Biophys Acta. 2013; 1831:1412–1425.33. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012; 14:5–14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells
- Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity
- Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation
- MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling
- Effect of Nitric Oxide on ADP-ribose Pyrophosphatase Activity