Lab Anim Res.
2011 Dec;27(4):317-325. 10.5625/lar.2011.27.4.317.
Inhibitory effect of Suaeda asparagoides (Miq.) extract on the motility of rat gastric antrum is mediated by beta-adrenoceptor
- Affiliations
-
- 1College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. twkim@mail.knu.ac.kr
- 2College of Pharmacy and Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu, Korea.
- 3Reclaimed Land Agriculture Research Division, National Institute of Crop Science, RDA, Iksan, Korea.
- 4Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 5Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 6Department of Physiology and Neuroscience, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
- KMID: 1444964
- DOI: http://doi.org/10.5625/lar.2011.27.4.317
Abstract
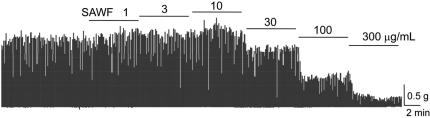
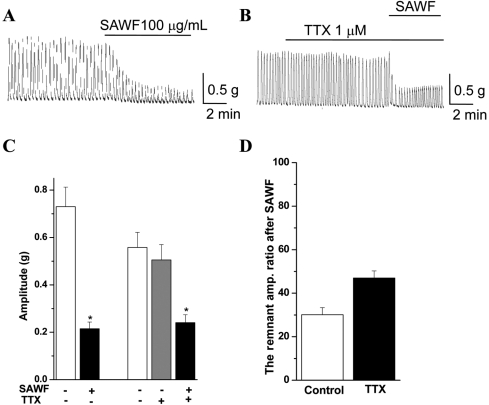
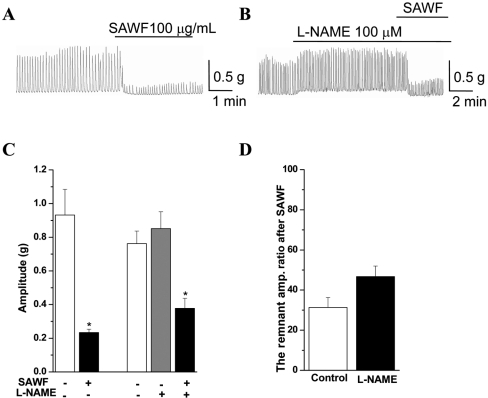
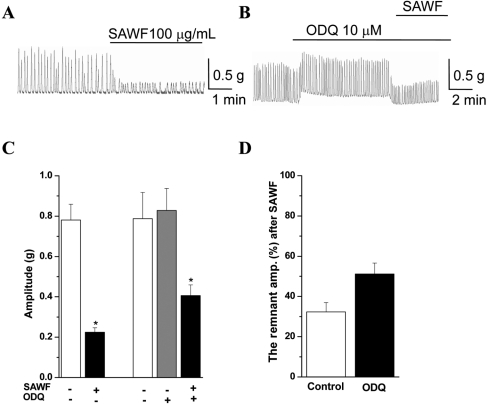
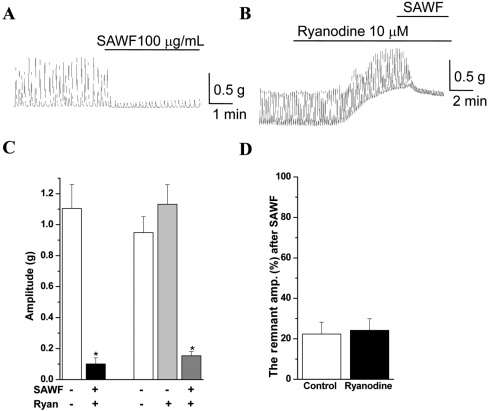
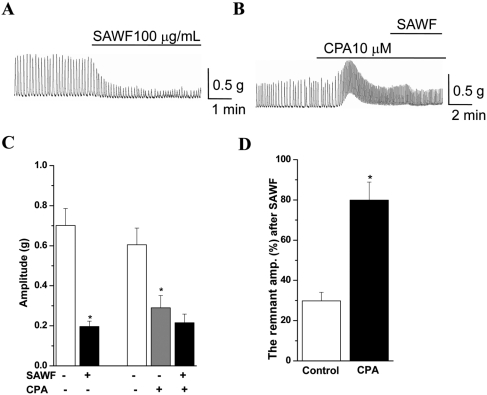
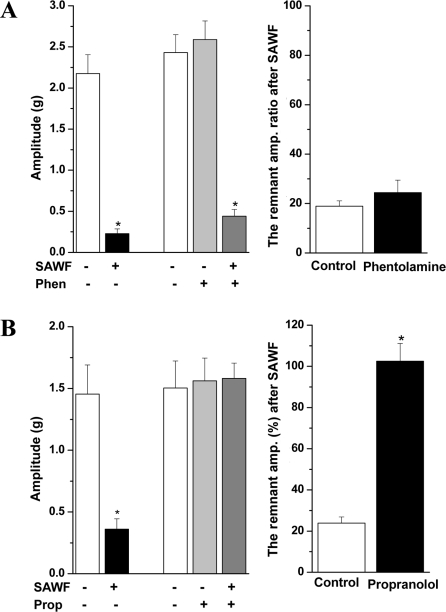
- Suaeda asparagoides (Miq.) has long been used as a Korean folk herbal medicine for the treatment of functional gastrointestinal disorders. However, reports on its pharmacological activity on gastrointestinal motility are scarce. The present study investigated the effects of Suaeda asparagoides water fraction of the extract (SAWF) on antral motility in vitro. Muscle strips from rat gastric antrum were set up in an organ bath in a circular orientation. SAWF (100 microg/mL) inhibited the spontaneous contraction of antral circular muscle strips. These inhibitory effects were not significantly affected by tetrodotoxin (1 microM), N omega-Nitro-L-arginine methyl ester hydrochloride (100 microM), 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (10 microM), ryanodine (10 microM) and phentolamine (10 microM). SAWF-induced inhibition was mostly restored by cyclopiazonic acid (10 microM). Furthermore, the beta-adrenergic receptor antagonist, propranolol (10 microM), abolished SAWF-induced inhibition. These results suggest that SAWF may exert its activity on gastrointestinal smooth muscle via a-adrenergic receptors and sarcoplasmic reticulum Ca2+ ATPase.
MeSH Terms
-
Animals
Baths
Calcium-Transporting ATPases
Carbamates
Chenopodiaceae
Contracts
Gastrointestinal Diseases
Gastrointestinal Motility
Herbal Medicine
Indoles
Muscle, Smooth
Muscles
Organometallic Compounds
Orientation
Oxadiazoles
Phentolamine
Propranolol
Pyloric Antrum
Quinoxalines
Rats
Ryanodine
Sarcoplasmic Reticulum
Tetrodotoxin
Water
Calcium-Transporting ATPases
Carbamates
Indoles
Organometallic Compounds
Oxadiazoles
Phentolamine
Propranolol
Quinoxalines
Ryanodine
Tetrodotoxin
Water
Figure
Reference
-
1. Szurszewski JH. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978; 277:91–102. PMID: 650594.
Article2. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.
Article3. Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol. 2006; 570(Pt 1):5–11. PMID: 16195319.
Article4. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997; 49(2):157–230. PMID: 9228665.5. Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005; 83(3):215–242. PMID: 15870837.
Article6. Gadano AB, Gurni AA, Carballo MA. Argentine folk medicine: genotoxic effects of Chenopodiaceae family. J Ethnopharmacol. 2006; 103(2):246–251. PMID: 16219440.7. Akah PA, Aguwa CN, Agu RU. Studies on the antidiarrhoeal properties of Pentaclethra macrophylla leaf extracts. Phytother Res. 1999; 13(4):292–295. PMID: 10404533.8. Amos S, Binda L, Kunle OF, Okafor I, Emeje M, Akah PA, Wambebe C, Gamaniel K. Smooth muscle contraction induced by Indigofera dendroides leaf extracts may involve calcium mobilization via potential sensitive channels. Phytother Res. 2003; 17(7):792–796. PMID: 12916079.9. Hohenester B, Ruhl A, Kelber O, Schemann M. The herbal preparation STW5 (lberogast) has potent and region-specific effects on gastric motility. Neurogastroenterol Motil. 2004; 16(6):765–773. PMID: 15601427.10. Roman-Ramos R, Flores-Saenz JL, Alarcon-Aguilar FJ. Anti-hyperglycemic effect of some edible plants. J Ethnopharmacol. 1995; 48(1):25–32. PMID: 8569244.
Article11. Park JM, Kim SD, Lee WM, Cho JY, Park HJ, Kim TW, Choe NH, Kim SK, Rhee MH. In vitro anti-oxidative and antiinflammatory effects of solvent-extracted fractions from Suaeda asparagoides. Pharmazie. 2007; 62(6):453–458. PMID: 17663194.12. Kim TW, Beckett EA, Hanna R, Koh SD, Ordog T, Ward SM, Sanders KM. Regulation of pacemaker frequency in the murine gastric antrum. J Physiol. 2002; 538(Pt 1):145–157. PMID: 11773323.
Article13. Amos S, Binda L, Chindo B, Akah P, Abdurahman M, Danmallam HU, Wambebe C, Gamaniel K. Evaluation of methanolic extract of Ficus platyphylla on gastrointestinal activity. Indian J Exp Biol. 2001; 39(1):63–67. PMID: 11349528.14. Gilani AH, Aziz N, Ali SM, Saeed M. Pharmacological basis for the use of peach leaves in constipation. J Ethnopharmacol. 2000; 73(1-2):87–93. PMID: 11025143.
Article15. Liu YH, Li ML, Hsu MY, Pang YY, Chen IL, Chen CK, Tang SW, Lin HY, Lin JY. Effects of a Chinese herbal medicine, Guan-Jen-Huang (Aeginetia indica Linn.), on renal cancer cell growth and metastasis. Evid Based Complement Alternat Med. 2012; in press.16. Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu IU. Investigations of the methanolic leaf extract of Costus afer. Ker for pharmacological activities in vitro and in vivo. Phytomedicine. 2004; 11(2-3):242–248. PMID: 15070179.17. Chang JY, Yang TY, Chang CP, Chang JG. The effect of "chi-han (hot nature)" Chinese herbs on the secretion of IL-1β and TNF-α by mononuclear cells. Kaohsiung J Med Sci. 1996; 12(1):18–24. PMID: 8871284.18. Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil. 2005; 17(5):643–653. PMID: 16185302.
Article19. Wang Y, Kondo T, Suzukamo Y, Oouchida Y, S . Izumi. Vagal nerve regulation is essential for the increase in gastric motility in response to mild exercise. Tohoku J Exp Med. 2010; 222(2):155–163. PMID: 20948179.20. Kawachi M, Matsunaga Y, Tanaka T, Hori Y, Ito K, Nagahama K, Ozaki T, Inoue N, Toda R, Yoshii K, Hirayama M, Kawabata Y, Takei M. Acotiamide hydrochloride (Z-338) enhances gastric motility and emptying by inhibiting acetylcholinesterase activity in rats. Eur J Pharmacol. 2011; 666(1-3):218–225. PMID: 21651906.
Article21. Toyomasu Y, Mochiki E, Yanai M, Ogata K, Tabe Y, Ando H, Ohno T, Aihara R, Zai H, Kuwano H. Intragastric monosodium L-glutamate stimulates motility of upper gut via vagus nerve in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R1125–R1135. PMID: 20071606.22. Kim T, La J, Lee J, Yang I. Effects of nitric oxide on slow waves and spontaneous contraction of guinea pig gastric antral circular muscle. J Pharmacol Sci. 2003; 92(4):337–347. PMID: 12939518.
Article23. Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992; 262(3 Pt 1):G379–G392. PMID: 1347974.
Article24. Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993; 1178(2):153–175. PMID: 7688574.
Article25. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525(Pt 2):355–361. PMID: 10835039.
Article26. Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl- conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009; 587(Pt 20):4905–4918. PMID: 19703958.27. Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004; 56(4):439–513. PMID: 15602008.
Article28. Bojo L, Nellgard P, Cassuto J. Effects of selective adrenergic agonists and antagonists on gastric tone in the rat. Acta Physiol Scand. 1991; 142(4):517–522. PMID: 1683093.29. Manara L, Croci T, Aureggi G, Guagnini F, Maffrand JP, Le Fur G, Mukenge S, Ferla G. Functional assessment of â adrenoceptor subtypes in human colonic circular and longitudinal (taenia coli) smooth muscle. Gut. 2000; 47(3):337–342. PMID: 10940268.30. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. Functional and molecular evidence for β1-, β2- and β-3-adrenoceptors in human colon. Br J Pharmacol. 1997; 120(8):1527–1535. PMID: 9113375.
Article31. Tanaka Y, Horinouchi T, Koike K. New insights into β-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol. 2005; 32(7):503–514. PMID: 16026507.
Article32. Colyer J. Phosphorylation states of phospholamban. Ann N Y Acad Sci. 1998; 853:79–91. PMID: 10603938.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of propofol on beta-adrenoceptor-mediated signal transduction in cardiac muscle; role of cAMP
- Effect of DA-9701 on the Normal Motility and Clonidine-induced Hypomotility of the Gastric Antrum in Rats
- Involvement of α1B -adrenoceptors and Rho kinase in contractions of rat aorta and mouse spleen
- Electroacupuncture ameliorates experimental colitis induced by acetic acid in rat
- Effects of Ethanol on the Motility of Isolated Strips of Antrum and Duodenum of the Rabbit