Korean J Physiol Pharmacol.
2023 Jul;27(4):325-331. 10.4196/kjpp.2023.27.4.325.
Involvement of α1B -adrenoceptors and Rho kinase in contractions of rat aorta and mouse spleen
- Affiliations
-
- 1Department of Physiology, King Abdulaziz University, Jeddah 22254, Saudi Arabia
- 2Department of Physiology, Royal College of Surgeons in Ireland, Dublin 2, D02 YN77, Ireland
- KMID: 2544134
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.325
Abstract
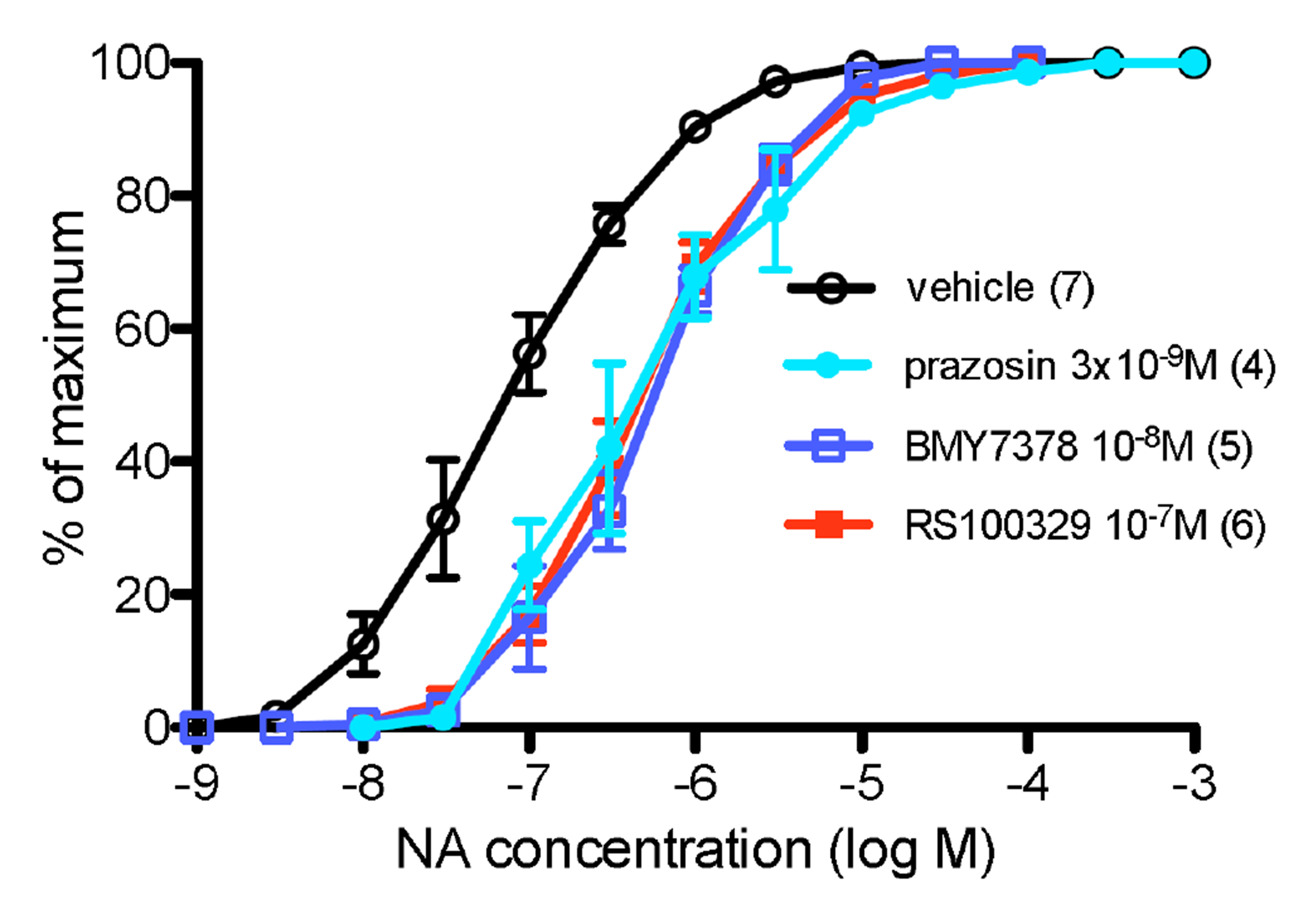
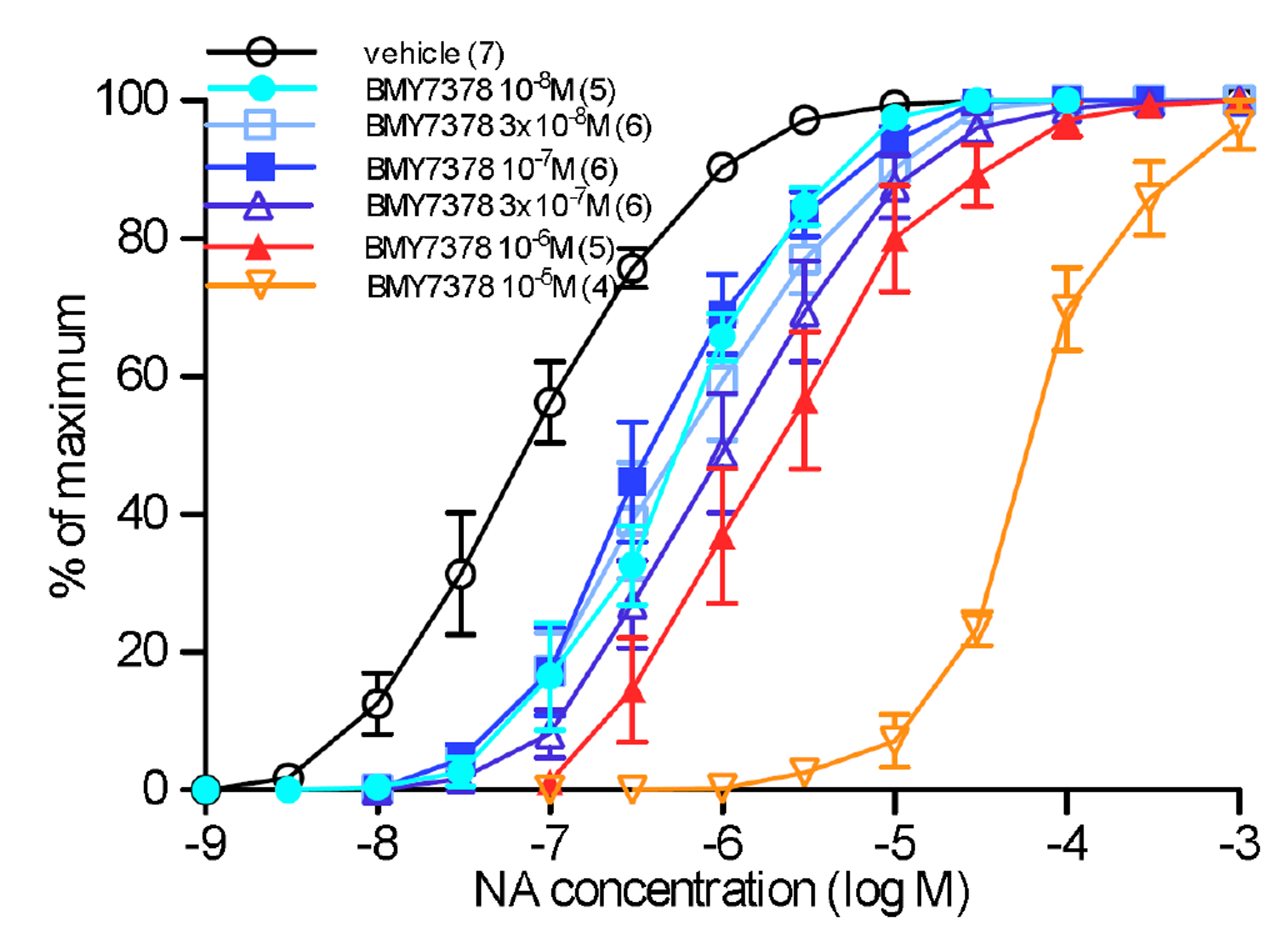
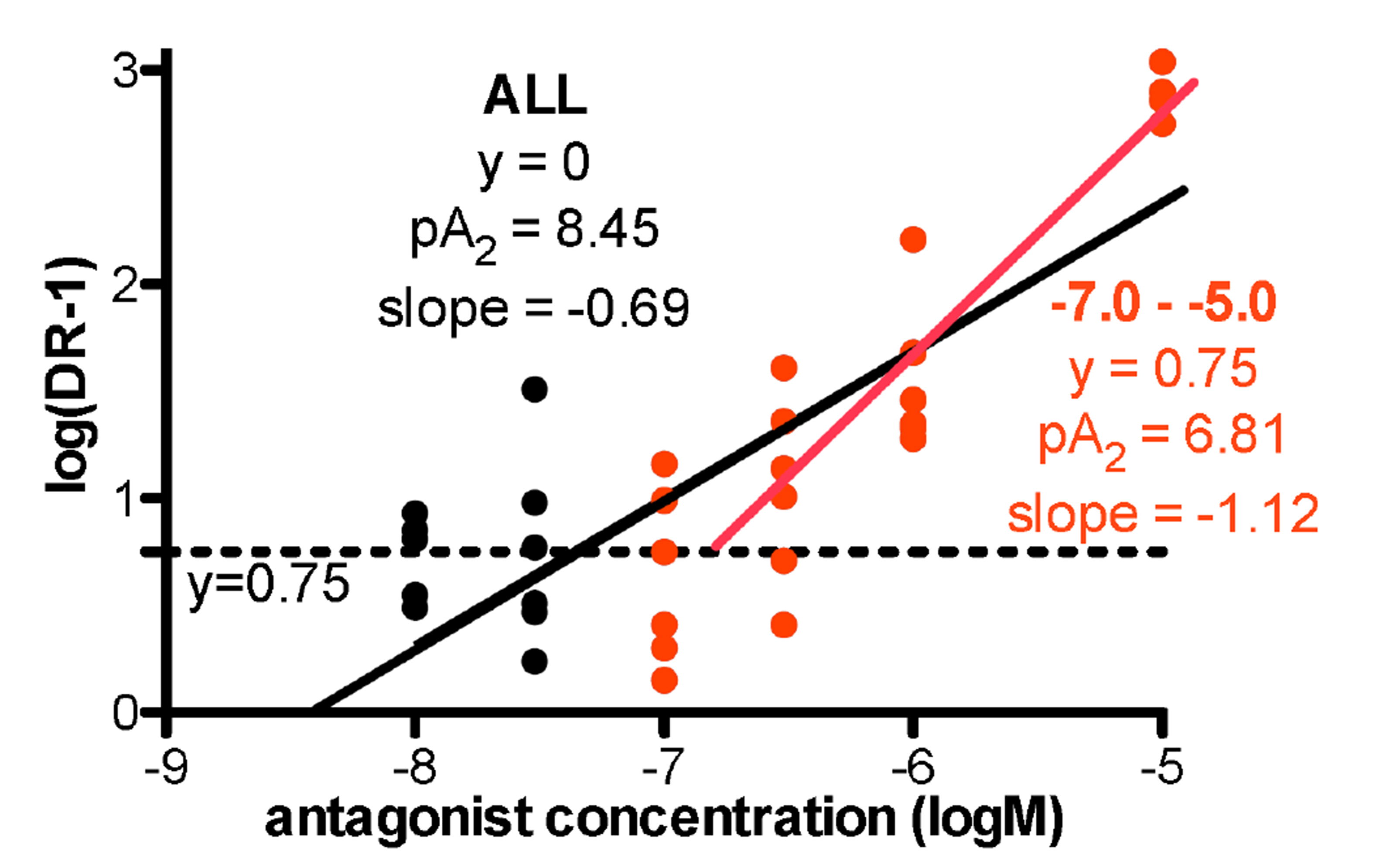
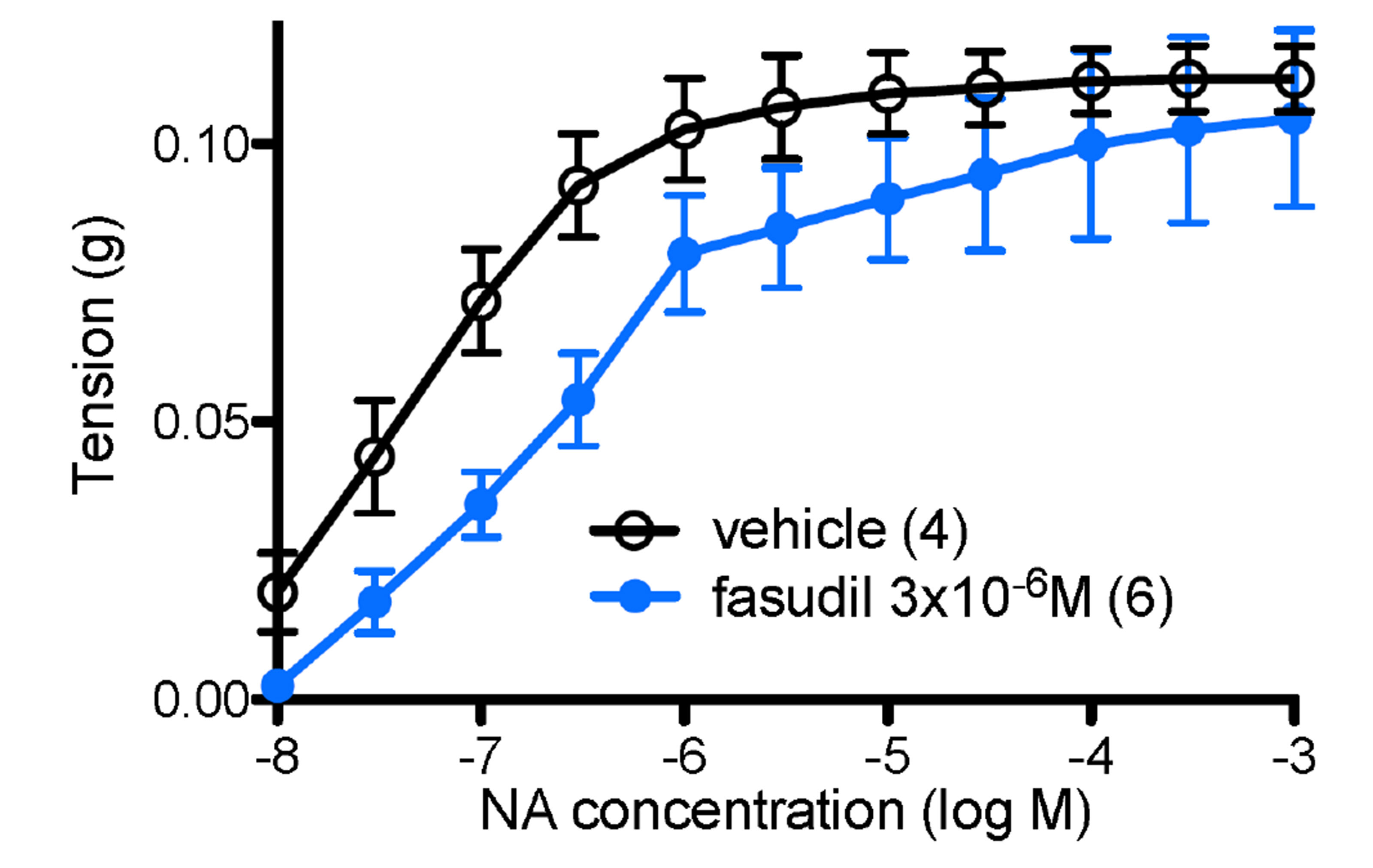
- α 1 -adrenoceptors link via the G-protein Gq/G11 to both Ca2+ entry and release from stores, but may also activate Rho kinase, which causes calcium sensitization. This study aimed to identify the subtype(s) of α1 -adrenoceptor involved in Rho kinase-mediated responses in both rat aorta and mouse spleen, tissues in which contractions involve multiple subtypes of α1 -adrenoceptor. Tissues were contracted with cumulative concentrations of noradrenaline (NA) in 0.5 log unit increments, before and in the presence of an antagonist or vehicle. Contractions produced by NA in rat aorta are entirely α1 -adrenoceptor mediated as they are competitively blocked by prazosin. The α1A -adrenoceptor antagonist RS100329 had low potency in rat aorta. The α1D -adrenoceptor antagonist BMY7378 antagonized contractions in rat aorta in a biphasic manner: low concentrations blocking α1D -adrenoceptors and high concentrations blocking α1B -adrenoceptors. The Rho kinase inhibitor fasudil (10 µM) significantly reduced aortic contractions in terms of maximum response, suggesting inhibition of α1B -adrenoceptor mediated responses. In the mouse spleen, a tissue in which all 3 subtypes of α1 -adrenoceptor are involved in contractions to NA, fasudil (3 µM) significantly reduced both early and late components to the NA contraction, the early component involving α1B - and α1D -adrenoceptors, and the late component involving α1B - and α1A -adrenoceptors. This suggests that fasudil inhibits α1B -adrenoceptor mediated responses. It is concluded that α1D - and α1B -adrenoceptors interact in rat aorta and α1D -, α1A - and α1B -adrenoceptors interact in the mouse spleen to produce contractions and these interactions suggest that one of the receptors preferentially activates Rho kinase, most likely the α1B -adrenoceptor.
Keyword
Figure
Reference
-
1. Harraz OF, Jensen LJ. 2021; Vascular calcium signalling and ageing. J Physiol. 599:5361–5377. DOI: 10.1113/JP280950. PMID: 34705288. PMCID: PMC9002240.
Article2. Fan G, Cui Y, Gollasch M, Kassmann M. 2019; Elementary calcium signaling in arterial smooth muscle. Channels (Austin). 13:505–519. DOI: 10.1080/19336950.2019.1688910. PMID: 31797713. PMCID: PMC6930021.
Article3. Shimokawa H, Sunamura S, Satoh K. 2016; RhoA/Rho-kinase in the cardiovascular system. Circ Res. 118:352–366. DOI: 10.1161/CIRCRESAHA.115.306532. PMID: 26838319.
Article4. Szasz T, Webb RC. 2017; Rho-mancing to sensitize calcium signaling for contraction in the vasculature: role of Rho kinase. Adv Pharmacol. 78:303–322. DOI: 10.1016/bs.apha.2016.09.001. PMID: 28212799.5. Nunes KP, Webb RC. 2021; New insights into RhoA/Rho-kinase signaling: a key regulator of vascular contraction. Small GTPases. 12:458–469. DOI: 10.1080/21541248.2020.1822721. PMID: 32970516. PMCID: PMC8583239.
Article6. Ergul M, Turgut NH, Sarac B, Altun A, Yildirim Ş, Bagcivan I. 2016; Investigating the effects of the Rho-kinase enzyme inhibitors AS1892802 and fasudil hydrochloride on the contractions of isolated pregnant rat myometrium. Eur J Obstet Gynecol Reprod Biol. 202:45–50. DOI: 10.1016/j.ejogrb.2016.04.031. PMID: 27160814.
Article7. Mochalov SV, Tarasova NV, Kudryashova TV, Gaynullina DK, Kalenchuk VU, Borovik AS, Vorotnikov AV, Tarasova OS, Schubert R. 2018; Higher Ca2+ -sensitivity of arterial contraction in 1-week-old rats is due to a greater Rho-kinase activity. Acta Physiol (Oxf). 223:e13044. DOI: 10.1111/apha.13044. PMID: 29383848.8. Docherty JR. 2019; The pharmacology of α1-adrenoceptor subtypes. Eur J Pharmacol. 855:305–320. DOI: 10.1016/j.ejphar.2019.04.047. PMID: 31067439.9. Ahmed A, Fusi F, Valoti M. 2022; Perivascular adipose tissue modulates the effects of flavonoids on rat aorta rings: role of superoxide anion and β3 receptors. Pharmacol Res. 180:106231. DOI: 10.1016/j.phrs.2022.106231. PMID: 35462011.10. Terada Y, Yayama K. 2021; Angiotensin II-induced vasoconstriction via Rho kinase activation in pressure-overloaded rat thoracic aortas. Biomolecules. 11:1076. DOI: 10.3390/biom11081076. PMID: 34439742. PMCID: PMC8391281. PMID: 34165df9e2394e6d9c78fa28973d958a.
Article11. Ishida H, Saito SY, Hishinuma E, Kitayama T, Ishikawa T. 2018; Differential contribution of calcium channels to α1-adrenoceptor-mediated contraction is responsible for diverse responses to cooling between rat tail and iliac arteries. Eur J Pharmacol. 826:9–16. DOI: 10.1016/j.ejphar.2018.02.023. PMID: 29458039.
Article12. Chung YH, Oh KW, Kim ST, Park ES, Je HD, Yoon HJ, Sohn UD, Jeong JH, La HO. 2018; Hypothermia inhibits endothelium-independent vascular contractility via Rho-kinase inhibition. Biomol Ther (Seoul). 26:139–145. DOI: 10.4062/biomolther.2016.233. PMID: 28208012. PMCID: PMC5839492.
Article13. Malacarne PF, Bezzenberger J, Lopez M, Warwick T, Müller N, Brandes RP, Rezende F. 2022; Epoxyeicosatrienoic acid and prostanoid crosstalk at the receptor and intracellular signaling levels to maintain vascular tone. Int J Mol Sci. 23:5939. DOI: 10.3390/ijms23115939. PMID: 35682616. PMCID: PMC9180422. PMID: 8c36df0c07b74a5e9bb249e648c8008b.
Article14. Alsufyani HA, Daly C, Docherty JR. 2021; Interaction between α1B - and other α1 - and α2 -adrenoceptors in producing contractions of mouse spleen. Basic Clin Pharmacol Toxicol. 129:416–426. DOI: 10.1111/bcpt.13639. PMID: 34383990.
Article15. Alsufyani HA, McCormick PA, Docherty JR. 2021; Both α1B- and α1A-adrenoceptor subtypes are involved in contractions of rat spleen. Pharmacol Rep. 73:255–260. DOI: 10.1007/s43440-020-00118-x. PMID: 32860192.
Article16. Hussain MB, Marshall I. 1997; Characterization of α1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 122:849–858. DOI: 10.1038/sj.bjp.0701461. PMID: 9384500. PMCID: PMC1565016.
Article17. O’Rourke M, Gavin K, Docherty JR. 1997; Further investigation of the alpha-adrenoceptor-mediated actions of chloroethylclonidine in rat aorta. Eur J Pharmacol. 336:37–42. DOI: 10.1016/S0014-2999(97)01257-0. PMID: 9384252.18. Alsufyani HA, Docherty JR. 2021; Involvement of G proteins and Rho kinase in α1-adrenoceptor mediated contractions of the rat portal vein. Can J Physiol Pharmacol. 99:654–659. DOI: 10.1139/cjpp-2020-0347. PMID: 33096009.
Article19. Martí D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, Moreno L, Villagrasa V, Barettino D, D’Ocon P. 2005; Correlation between mRNA levels and functional role of α1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the α1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 289:H1923–H1932. DOI: 10.1152/ajpheart.00288.2005. PMID: 15951348.20. Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, Cotecchia S, Kitamura T, Tsujimoto G, Koike K. 2005; Correlation between vasoconstrictor roles and mRNA expression of α1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol. 146:456–466. DOI: 10.1038/sj.bjp.0706325. PMID: 16113694. PMCID: PMC1576278.
Article21. Weinberg DH, Trivedi P, Tan CP, Mitra S, Perkins-Barrow A, Borkowski D, Strader CD, Bayne M. 1994; Cloning, expression and characterization of human alpha adrenergic receptors alpha 1a, α1B and α1c. Biochem Biophys Res Commun. 201:1296–1304. DOI: 10.1006/bbrc.1994.1845. PMID: 8024574.
Article22. Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione C, Mostardini M, Schmidt A, Beermann F, Cotecchia S. 1997; Decreased blood pressure response in mice deficient of the α1B-adrenergic receptor. Proc Natl Acad Sci U S A. 94:11589–11594. DOI: 10.1073/pnas.94.21.11589. PMID: 9326654. PMCID: PMC23548.
Article23. Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. 2002; The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 109:765–775. DOI: 10.1172/JCI200214001. PMID: 11901185. PMCID: PMC150908.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roles for α1-adrenoceptors during contractions by electrical field stimulation in mouse vas deferens
- C-Jun NH2-Terminal Kinase Contributes to Dexmedetomidine-Induced Contraction in Isolated Rat Aortic Smooth Muscle
- Endothelium Independent Effect of Pelargonidin on Vasoconstriction in Rat Aorta
- Flavone Attenuates Vascular Contractions by Inhibiting RhoA/Rho Kinase Pathway
- Effect of Tetrahydroisoquinoline Alkaloids on the alpha-adrenoceptors in Rat Aorta and Brain Homogenates