Korean J Physiol Pharmacol.
2021 Nov;25(6):525-532. 10.4196/kjpp.2021.25.6.525.
Roles for α1-adrenoceptors during contractions by electrical field stimulation in mouse vas deferens
- Affiliations
-
- 1Department of Physiology, King Abdulaziz University, Jeddah 21589, Saudi Arabia
- 2Department of Physiology, Royal College of Surgeons in Ireland (RCSI), Dublin D02 YN77, Ireland
- KMID: 2521478
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.525
Abstract
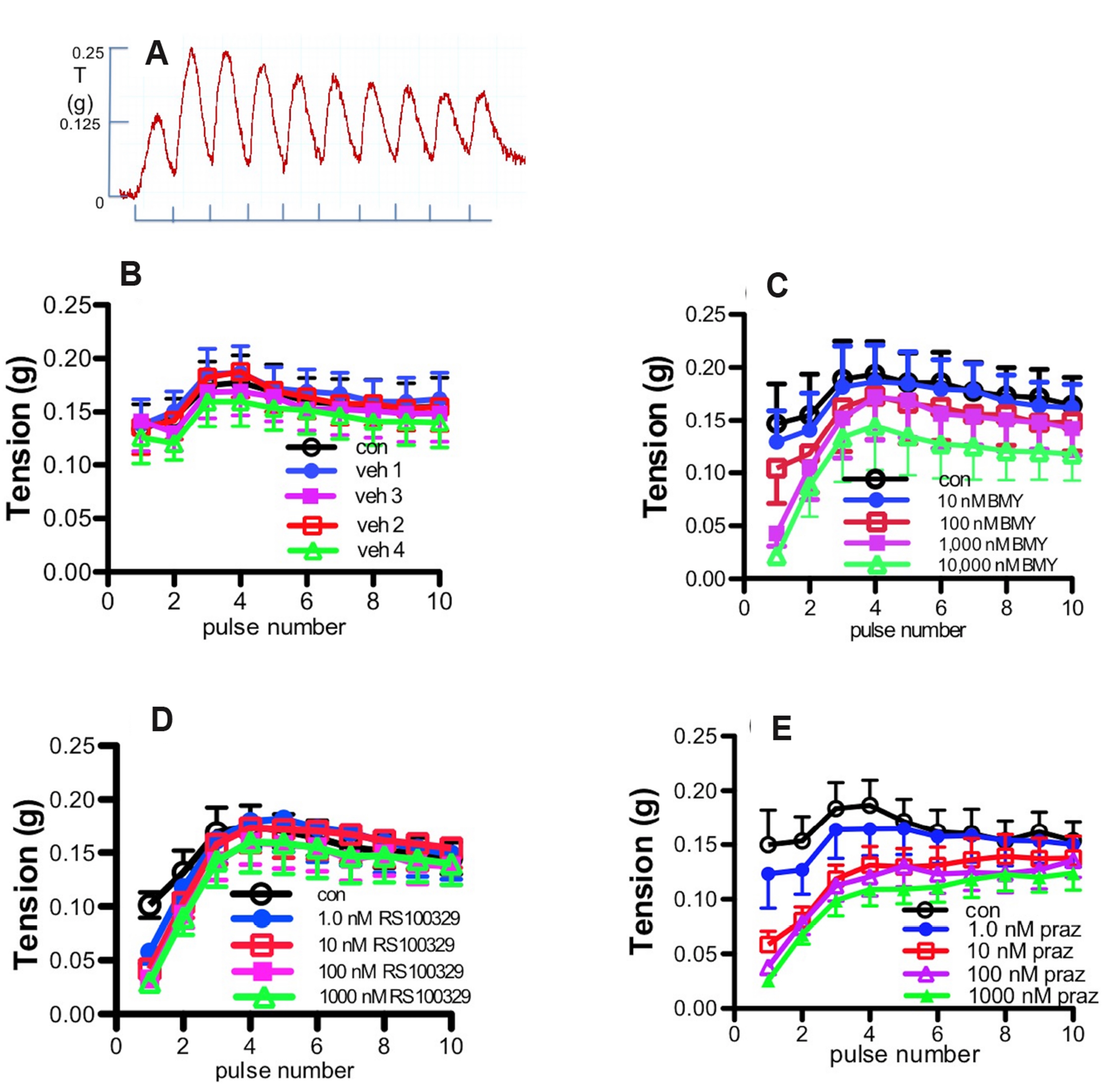
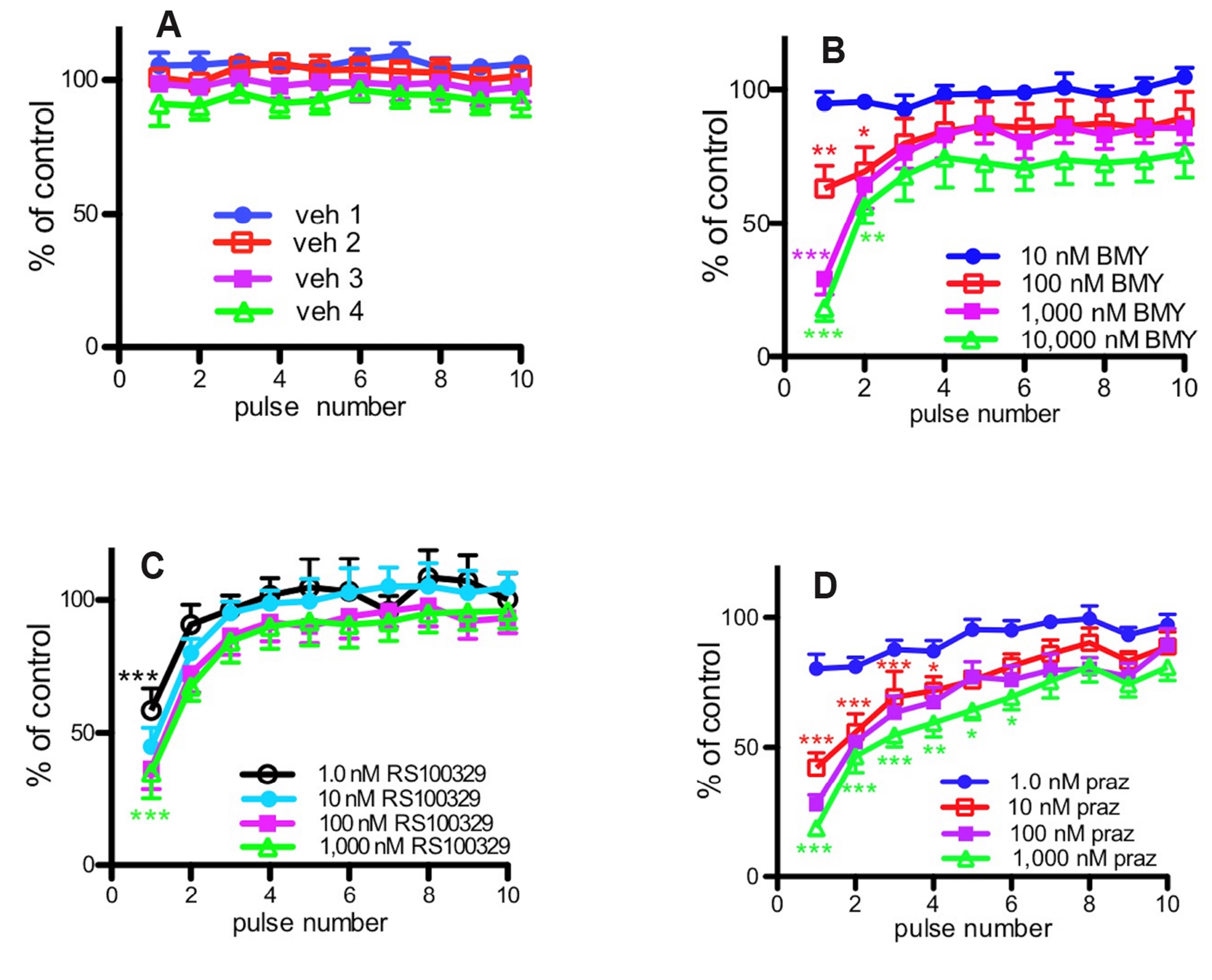
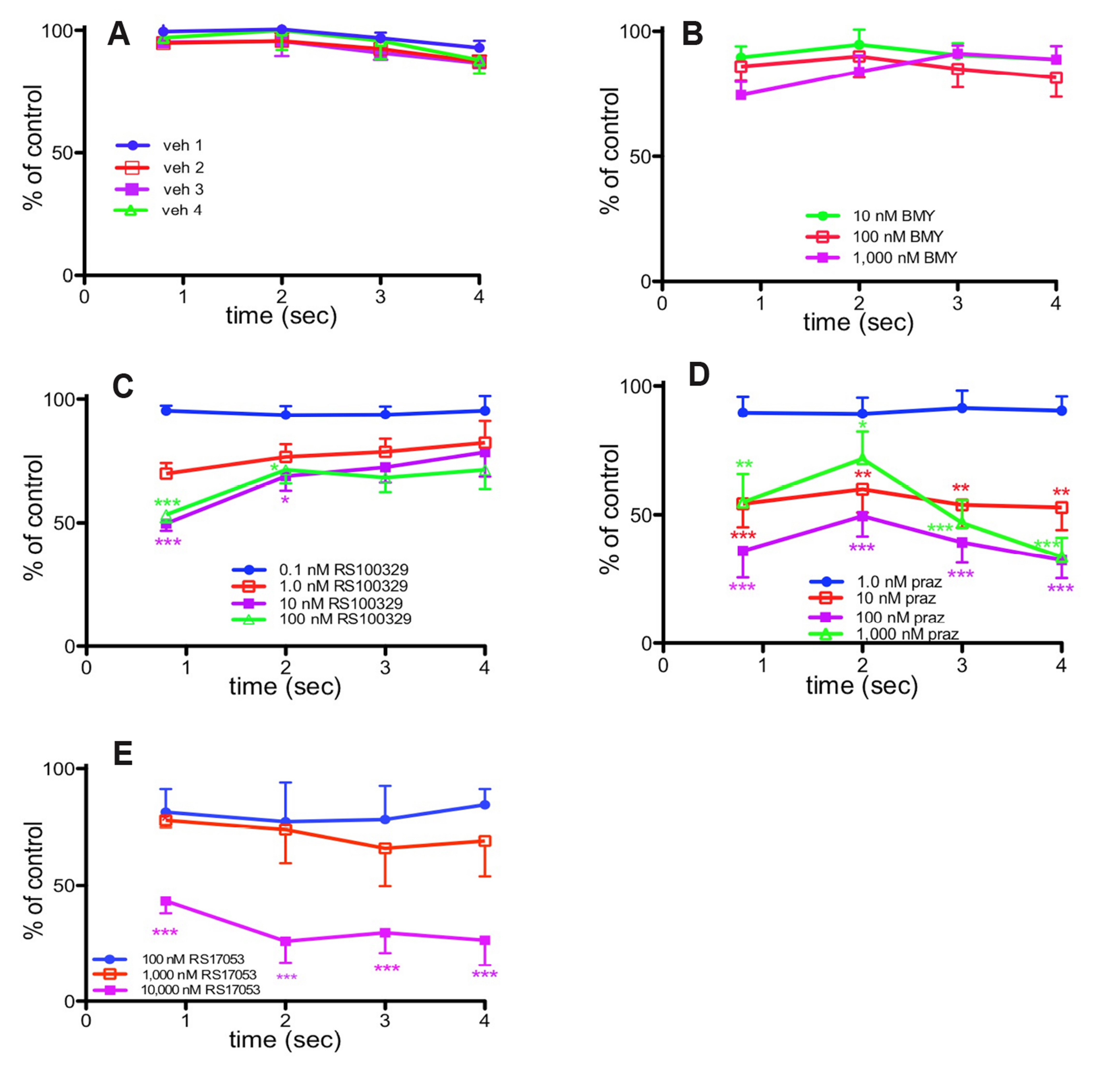
- We have investigated the relative roles of α1-adrenoceptors and purinoceptors in contractions to low and high frequency stimulation of the mouse vas deferens, in terms of the time course of responses. In separate experiments, isometric contractile responses were obtained to 10 pulses at 1 Hz and 40 pulses at 10 Hz. Responses to 1 Hz stimulation consisted of a series of discrete peaks. The α1A -adrenoceptor antagonist RS100329 (10 –9 M–10 –7 M) significantly reduced the response to the first pulse, the α1D-adrenoceptor antagonist BMY7378 (10 –7 M–10–6 M) significantly reduced the response to the first two pulses, and the non-selective α1-adrenoceptor antagonist prazosin (10–8 M) reduced the response to the first 4 pulses at 1 Hz. Responses to 10 Hz stimulation consisted of an early peak response and a maintained plateau response. RS100329 significantly reduced the peak response but did not significantly affect the plateau response. Prazosin, significantly reduced both the peak and plateau responses. The α1A-adrenoceptor antagonist RS17053 in high concentrations reduced mainly the plateau response leaving a clear early peak response. The plateau response of contraction was almost abolished by the purinoceptor antagonist suramin. These results suggest that there is a relatively minor early α1D -adrenoceptor and a larger early α1A -adrenoceptor component to stimulationevoked contractions of mouse vas deferens, but the major α1 -adrenoceptor component is revealed by prazosin to be α1B -adrenoceptor mediated. α1B -Adrenoceptor activation probably facilitates contractions mediated by other α1-adrenoceptors and by purinoceptors. These results suggest that combined non-selective α1-adrenoceptor blockade, particularly α1B -adrenoceptor blockade, in addition to P2X1-purinoceptor blockade is useful in reducing male fertility.
Keyword
Figure
Reference
-
1. Young JS, Brain KL, Cunnane TC. 2007; Electrical and optical study of nerve impulse-evoked ATP-induced, P2X-receptor-mediated sympathetic neurotransmission at single smooth muscle cells in mouse isolated vas deferens. Neuroscience. 148:82–91. DOI: 10.1016/j.neuroscience.2007.05.044. PMID: 17629625. PMCID: PMC2151008.
Article2. Bexis S, Cleary L, McGrath JC, Tanoue A, Tsujimoto G, Docherty JR. 2008; Alpha1D-adrenoceptors mediate nerve and agonist-evoked contractions in mouse vas deferens: evidence obtained from knockout technology. Auton Autacoid Pharmacol. 28:81–85. DOI: 10.1111/j.1474-8673.2008.00420.x. PMID: 18598289.3. Docherty JR. 2013; Prazosin has low potency at α1A-adrenoceptors and high potency at α1D-adrenoceptors in rat vas deferens. Auton Autacoid Pharmacol. 33:49–57. DOI: 10.1111/aap.12015. PMID: 24074250.4. Gorodeski GI. 2015; Purinergic signalling in the reproductive system. Auton Neurosci. 191:82–101. DOI: 10.1016/j.autneu.2015.04.008. PMID: 25981553.
Article5. White CW, Choong YT, Short JL, Exintaris B, Malone DT, Allen AM, Evans RJ, Ventura S. 2013; Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice. Proc Natl Acad Sci U S A. 110:20825–20830. DOI: 10.1073/pnas.1318624110. PMID: 24297884. PMCID: PMC3870707.6. Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Gever JR, Kava MS, Morgans D, Padilla F, Tassa S, Vimont RL, Chapple CR, Chess-Williams R, Eglen RM, Clarke DE, Ford AP. 1999; In vitro α1-adrenoceptor pharmacology of Ro 70-0004 and RS-100329, novel α1A -adrenoceptor selective antagonists. Br J Pharmacol. 127:252–258. DOI: 10.1038/sj.bjp.0702541. PMID: 10369480. PMCID: PMC1566006.7. Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL Jr. 1995; BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur J Pharmacol. 272:R5–R6. DOI: 10.1016/0014-2999(94)00751-R.8. Docherty JR. 2019; The pharmacology of α1-adrenoceptor subtypes. Eur J Pharmacol. 855:305–320. https://doi.org/10.1016/j.ejphar.2019.04.047. DOI: 10.1016/j.ejphar.2019.04.047. PMID: 31067439.
Article9. Kenny BA, Miller AM, Williamson IJ, O'Connell J, Chalmers DH, Naylor AM. 1996; Evaluation of the pharmacological selectivity profile of α1 adrenoceptor antagonists at prostatic α1 adrenoceptors: binding, functional and in vivo studies. Br J Pharmacol. 118:871–878. DOI: 10.1111/j.1476-5381.1996.tb15480.x. PMID: 8799556. PMCID: PMC1909506.10. Alsufyani HA, Docherty JR. 2021; Involvement of G proteins and Rho kinase in α1-adrenoceptor mediated contractions of the rat portal vein. Can J Physiol Pharmacol. 99:654–659. DOI: 10.1139/cjpp-2020-0347. PMID: 33096009.11. Alsufyani HA, McCormick PA, Docherty JR. 2021; Both α1B- and α1A-adrenoceptor subtypes are involved in contractions of rat spleen. Pharmacol Rep. 73:255–260. DOI: 10.1007/s43440-020-00118-x. PMID: 32860192.12. Cleary L, Slattery J, Bexis S, Docherty JR. 2004; Sympathectomy reveals α1A- and α1D-adrenoceptor components to contractions to noradrenaline in rat vas deferens. Br J Pharmacol. 143:745–752. DOI: 10.1038/sj.bjp.0705987. PMID: 15451776. PMCID: PMC1575931.13. Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, Clarke DE. 1997; Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 121:1127–1135. DOI: 10.1038/sj.bjp.0701207. PMID: 9249248. PMCID: PMC1564783.14. Marshall I, Burt RP, Green GM, Hussain MB, Chapple CR. 1996; Different subtypes of α1A-adrenoceptor mediating contraction of rat epididymal vas deferens, rat hepatic portal vein and human prostate distinguished by the antagonist RS 17053. Br J Pharmacol. 119:407–415. DOI: 10.1111/j.1476-5381.1996.tb16001.x. PMID: 8886428. PMCID: PMC1915842.15. Seto SW, Bexis S, McCormick PA, Docherty JR. 2010; Actions of thalidomide in producing vascular relaxations. Eur J Pharmacol. 644:113–119. DOI: 10.1016/j.ejphar.2010.06.035. PMID: 20615401.
Article16. Bültmann R, Trendelenburg M, Tuluc F, Wittenburg H, Starke K. 1999; Concomitant blockade of P2X-receptors and ecto-nucleotidases by P2-receptor antagonists: functional consequences in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 359:339–344. DOI: 10.1007/PL00005360. PMID: 10344533.
Article17. Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. 2000; Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 403:86–89. DOI: 10.1038/47495. PMID: 10638758.
Article18. Chiba Y, Misawa M. 2004; The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J Smooth Muscle Res. 40:155–167. DOI: 10.1540/jsmr.40.155. PMID: 15655303.19. Perez DM, Piascik MT, Graham RM. 1991; Solution-phase library screening for the identification of rare clones: isolation of an α1D-adrenergic receptor cDNA. Mol Pharmacol. 40:876–883.20. Yono M, Tanaka T, Tsuji S, Hori M, Irie S, Sakata Y, Otani M, Yoshida M, Latifpour J. 2012; A comparison of the expression and contractile function of α1-adrenoceptors in seminal vesicle and vas deferens from normotensive and hypertensive rats. Eur J Pharmacol. 694:104–110. DOI: 10.1016/j.ejphar.2012.08.005. PMID: 22960063.21. Cleary L, Vandeputte C, Docherty JR. 2002; Investigation of neurotransmission in vas deferens from α2A/D-adrenoceptor knockout mice. Br J Pharmacol. 136:857–864. DOI: 10.1038/sj.bjp.0704791. PMID: 12110610. PMCID: PMC1573420.22. Sanbe A, Tanaka Y, Fujiwara Y, Tsumura H, Yamauchi J, Cotecchia S, Koike K, Tsujimoto G, Tanoue A. 2007; Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol. 152:332–340. DOI: 10.1038/sj.bjp.0707366. PMID: 17603545. PMCID: PMC2042949.23. Paz GF, Shilon M, Homonnai ZT. 1984; The possible use of phenoxybenzamine as a male contraceptive drug: studies on male rats. Contraception. 29:189–195. DOI: 10.1016/0010-7824(84)90029-5. PMID: 6723312.
Article24. Homonnai ZT, Shilon M, Paz GF. 1984; Phenoxybenzamine--an effective male contraceptive pill. Contraception. 29:479–491. DOI: 10.1016/0010-7824(84)90022-2. PMID: 6430643.25. Ratnasooriya WD, Wadsworth RM. 1990; Impairment of fertility of male rats with prazosin. Contraception. 41:441–447. DOI: 10.1016/0010-7824(90)90043-U. PMID: 2335107.
Article26. Kjaergaard N, Kjaergaard B, Lauritsen JG. 1988; Prazosin, an adrenergic blocking agent inadequate as male contraceptive pill. Contraception. 37:621–629. DOI: 10.1016/0010-7824(88)90008-X. PMID: 2899490.
Article27. Mathiew M, Dennis BM, Bennetts F, Su NNE, Nguyen N, Botteon A, Baell JB, Ventura S. 2020; Synthesis of 2-phenyl-5,6,7,8-tetrahydroquinoxaline derivatives and screening for P2X1-purinoceptor antagonist activity in isolated preparations of rat vas deferens, for translation into a male contraceptive. Biol Reprod. 103:323–332. DOI: 10.1093/biolre/ioaa117. PMID: 32648904. PMCID: PMC7526726.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contractile Response of the Rat Vas Deferens to Electrical Field Stimulation, Phenylephrine and Acetylcholine under Hypoxia
- Changes of Contractility of the Vas Deferens to Field Stimulation in Diabetic Rats
- Effects of octreotide on the contractility of isolated rat vas deferens
- Role of Nitric Oxide in the Motor Activity of Rat Vas Deferens
- The Effects of Sympathectomy by 6-Hydroxydopamine and Pretreatment of Testosterone on the Contractility of the Vas Deferens in Rats