Korean J Physiol Pharmacol.
2013 Feb;17(1):15-21. 10.4196/kjpp.2013.17.1.15.
Influence of Aspirin on Pilocarpine-Induced Epilepsy in Mice
- Affiliations
-
- 1Department of Pharmacology, Cell Death Disease Research Center, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. syk@catholic.ac.kr
- 2Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 3Department of Pharmaceutical Science and Technology, The Catholic University of Daegu, Daegu 712-702, Korea.
- KMID: 1432758
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.15
Abstract
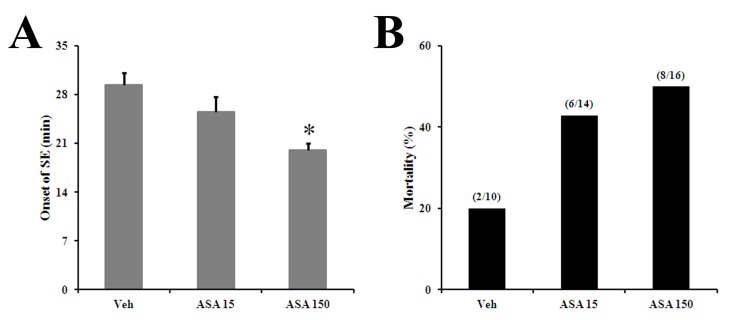
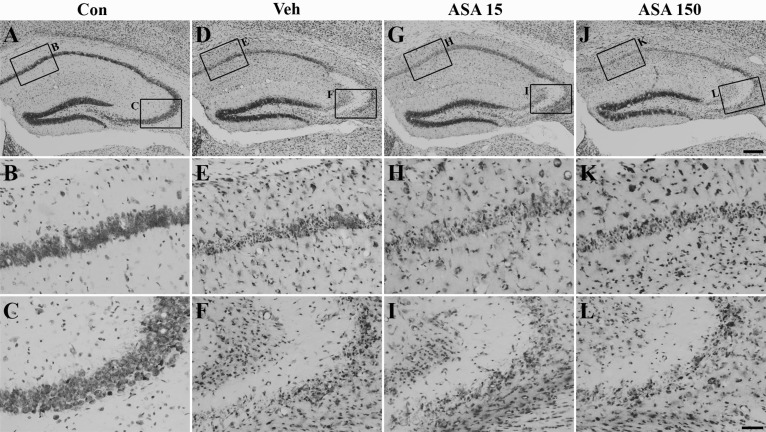
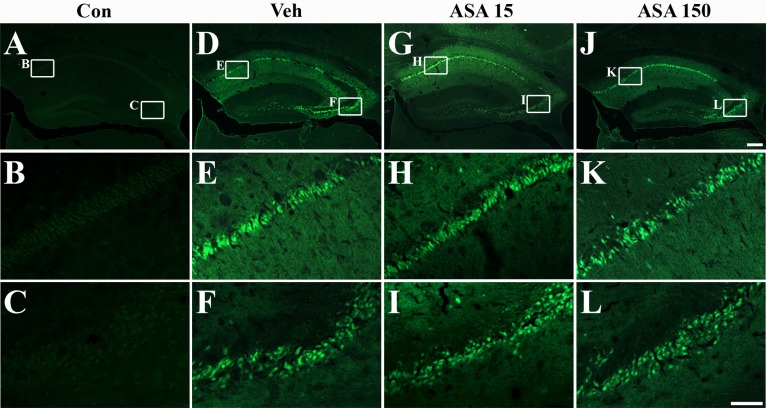
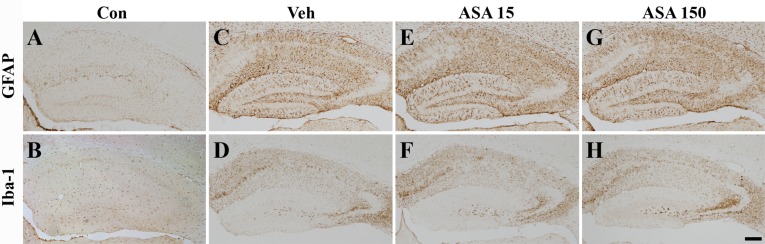
- Aspirin (acetylsalicylic acid) is one of the most widely used therapeutic agents based on its pharmacological actions, including anti-inflammatory, analgesic, anti-pyretic, and anti-thrombotic effects. In this study, we investigated the effects of aspirin on seizure susceptibility and hippocampal neuropathology following pilocarpine-induced status epilepticus (SE). SE was induced by pilocarpine hydrochloride (280 mg/kg, i.p.) administration in C57BL/6 mice (aged 8 weeks). Aspirin was administered daily (15 mg/kg or 150 mg/kg, i.p.) for 10 days starting 3 days before SE, continuing until 6 days after SE. After pilocarpine injection, SE onset time and mortality were recorded. Neuronal cell death was examined using cresyl violet and Fluoro-Jade staining, and glial responses were observed 7 days post SE using immunohistochemistry. In the aspirin-treated group, the onset time of SE was significantly shortened and mortality was markedly increased compared to the control group. However, in this study, aspirin treatment did not affect SE-induced neuronal cell death or astroglial and microglial responses in the hippocampus. In conclusion, these results suggest that the safety of aspirin should be reevaluated in some patients, especially with neurological disorders such as temporal lobe epilepsy.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Neuroprotective effect of lithium after pilocarpine-induced status epilepticus in mice
Namgue Hong, Yun-Sik Choi, Seong Yun Kim, Hee Jung Kim
Korean J Physiol Pharmacol. 2017;21(1):125-131. doi: 10.4196/kjpp.2017.21.1.125.Quantitative analysis of acetylsalicylic acid in human blood using volumetric absorptive microsampling
Yunjeong Kim, Ji-Young Jeon, Song-Hee Han, Na Ha, Kyungho Jang, Min-Gul Kim
Transl Clin Pharmacol. 2018;26(1):32-38. doi: 10.12793/tcp.2018.26.1.32.
Reference
-
1. Fisher PD, Sperber EF, Moshé SL. Hippocampal sclerosis revisited. Brain Dev. 1998; 20:563–573. PMID: 9865538.
Article2. Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002; 49:109–120. PMID: 12049799.
Article3. Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984; 321:237–253. PMID: 6498517.
Article4. Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res. 2002; 135:121–131. PMID: 12143334.
Article5. Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004; 54:473–485. PMID: 15575361.6. Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004; 73:1–60. PMID: 15193778.
Article7. McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006; 2006:re12. PMID: 17033045.
Article8. Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc. 2009; 81:345–365. PMID: 19722008.
Article9. Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011; 497:223–230. PMID: 21362451.
Article10. Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008; 29:142–160. PMID: 17931873.
Article11. Lehtimäki KA, Liimatainen S, Peltola J, Arvio M. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res. 2011; 95:184–187. PMID: 21530175.
Article12. Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011; 85:6913–6922. PMID: 21543484.
Article13. Auvin S, Mazarati A, Shin D, Sankar R. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis. 2010; 40:303–310. PMID: 20600912.
Article14. Pitkänen A. Therapeutic approaches to epileptogenesis--hope on the horizon. Epilepsia. 2010; 51(Suppl 3):2–17. PMID: 20618393.
Article15. Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011; 10:173–186. PMID: 21256455.
Article16. Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010; 62:668–700. PMID: 21079040.
Article17. Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002; 447:1–9. PMID: 12106797.
Article18. Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, Schnaper HW, LeWinter MM, Linares E, Pouget JM, Sabharwal SC, Chesler E, DeMots H. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983; 309:396–403. PMID: 6135989.19. Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012; 9:259–267. PMID: 22473097.
Article20. Bernheisel CR, Schlaudecker JD, Leopold K. Subacute management of ischemic stroke. Am Fam Physician. 2011; 84:1383–1388. PMID: 22230273.21. Ikonomidou-Turski C, Cavalheiro EA, Turski L, Bortolotto ZA, Kleinrok Z, Calderazzo-Filho LS, Turski WA. Differential effects of non-steroidal anti-inflammatory drugs on seizures produced by pilocarpine in rats. Brain Res. 1988; 462:275–285. PMID: 3191389.
Article22. Najbauer J, Schuman EM, Mamelak AN. The aspirin metabolite sodium salicylate causes focal cerebral hemorrhage and cell death in rats with kainic acid-induced seizures. Neuroscience. 2000; 99:107–117. PMID: 10924956.
Article23. Ma L, Cui XL, Wang Y, Li XW, Yang F, Wei D, Jiang W. Aspirin attenuates spontaneous recurrent seizures and inhibits hippocampal neuronal loss, mossy fiber sprouting and aberrant neurogenesis following pilocarpine-induced status epilepticus in rats. Brain Res. 2012; 1469:103–113. PMID: 22765917.
Article24. Tandon M, Anuradha K, Pandhi P. Evaluation of antiepileptic activity of aspirin in combination with newer antiepileptic lamotrigine in mice. Methods Find Exp Clin Pharmacol. 2003; 25:607–610. PMID: 14671677.
Article25. Mordenti J, Chappell W. Yacobi A, Skelly JP, Batra VK, editors. The use of interspecies scaling in toxicokinetics. Toxicokinetics and New Drug Development. 1989. New York: Pergamon Press;p. 42–96.26. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972; 32:281–294. PMID: 4110397.
Article27. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic coordinates. 2001. San Diego: Academic Press.28. Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003; 182:21–34. PMID: 12821374.
Article29. Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998; 56:149–171. PMID: 9760699.
Article30. Bruke A, Smyth EM, FitzGerald GA. Brunton LL, Lazo JS, Parker KL, editors. Analgesic-Antipyretic Agents; Pharmacotherapy of Goat. Goodman & Gilman's the pharmacological basis of therapeutics. 2006. 11th ed. New York: McGraw-Hill;p. 671–715.31. Schubert TA. Salicylate-induced seizures in a dog. J Am Vet Med Assoc. 1984; 185:1000–1001. PMID: 6511630.32. Chen CS, Aberdeen GC. Potentiation of acoustic-trauma-induced audiogenic seizure susceptibility by salicylates in mice. Experientia. 1980; 36:330–331. PMID: 7371791.
Article33. Sánchez-Hernandez MC, Delgado J, Navarro AM, Orta JC, Hernandez M, Conde J. Seizures induced by NSAID. Allergy. 1999; 54:90–91. PMID: 10195372.
Article34. Senol N, Ceyhan BM, Ersoy IH, Senol A, Acarturk G, Sutcu R. Aspirin increases NMDA receptor subunit 2A concentrations in rat hippocampus. J Recept Signal Transduct Res. 2012; 32:17–21. PMID: 22171557.
Article35. Croucher MJ, Collins JF, Meldrum BS. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982; 216:899–901. PMID: 7079744.
Article36. Bengzon J, Okabe S, Lindvall O, McKay RD. Suppression of epileptogenesis by modification of N-methyl-D-aspartate receptor subunit composition. Eur J Neurosci. 1999; 11:916–922. PMID: 10103085.37. Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007; 27:542–552. PMID: 17234586.
Article38. Wang SJ. Facilitatory effect of aspirin on glutamate release from rat hippocampal nerve terminals: involvement of protein kinase C pathway. Neurochem Int. 2006; 48:181–190. PMID: 16330128.
Article39. Lu YG, Tang ZQ, Ye ZY, Wang HT, Huang YN, Zhou KQ, Zhang M, Xu TL, Chen L. Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits. Br J Pharmacol. 2009; 157:1514–1522. PMID: 19594751.40. Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010; 91:49–56. PMID: 20643531.
Article41. Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999; 843:118–129. PMID: 10528118.
Article42. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006; 23:237–246. PMID: 16806953.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hippocampal Neurogenesis and Phenotypic Differentiation after Pilocarpine-Induced Seizures in Young Mice
- The Role of MMP-9 on the Hippocampal Neuronal Cell Death and Mossy Fiber Sprouting due to Pilocarpine-Induced Status Epilepticus in Mice
- Spatiotemporal expression of RCAN1 and its isoform RCAN1-4 in the mouse hippocampus after pilocarpine-induced status epilepticus
- Time-course changes of hippocalcin expression in the mouse hippocampus following pilocarpine-induced status epilepticus
- The Effect and the Mechanism of Action of High Frequency Electrical Stimulation of Subthalamic Nucleus on Status Epilepticus Induced by Lithium-Pilocarpine of Rat