Diabetes Metab J.
2020 Feb;44(1):67-77. 10.4093/dmj.2018.0274.
Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. injkim@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 3Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 4Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 5Department of Internal Medicine, Busan St. Mary's Hospital, Catholic University of Pusan, Busan, Korea.
- 6Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 7Department of Internal Medicine, Daedong Hospital, Busan, Korea.
- 8Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 9Department of Internal Medicine, Dong-A Medical Center, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2470956
- DOI: http://doi.org/10.4093/dmj.2018.0274
Abstract
- BACKGROUND
There is limited information regarding the optimal third-line therapy for managing type 2 diabetes mellitus (T2DM) that is inadequately controlled using dual combination therapy. This study assessed the efficacy and safety of pioglitazone or glimepiride when added to metformin plus alogliptin treatment for T2DM.
METHODS
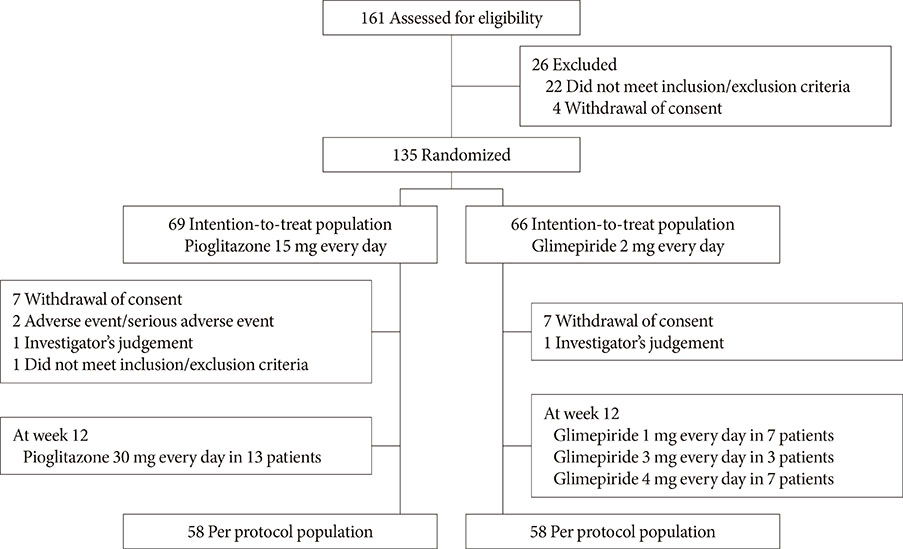
This multicenter, randomized, active-controlled trial (ClinicalTrials.gov: NCT02426294) recruited 135 Korean patients with T2DM that was inadequately controlled using metformin plus alogliptin. The patients were then randomized to also receive pioglitazone (15 mg/day) or glimepiride (2 mg/day) for a 26-week period, with dose titration was permitted based on the investigator's judgement.
RESULTS
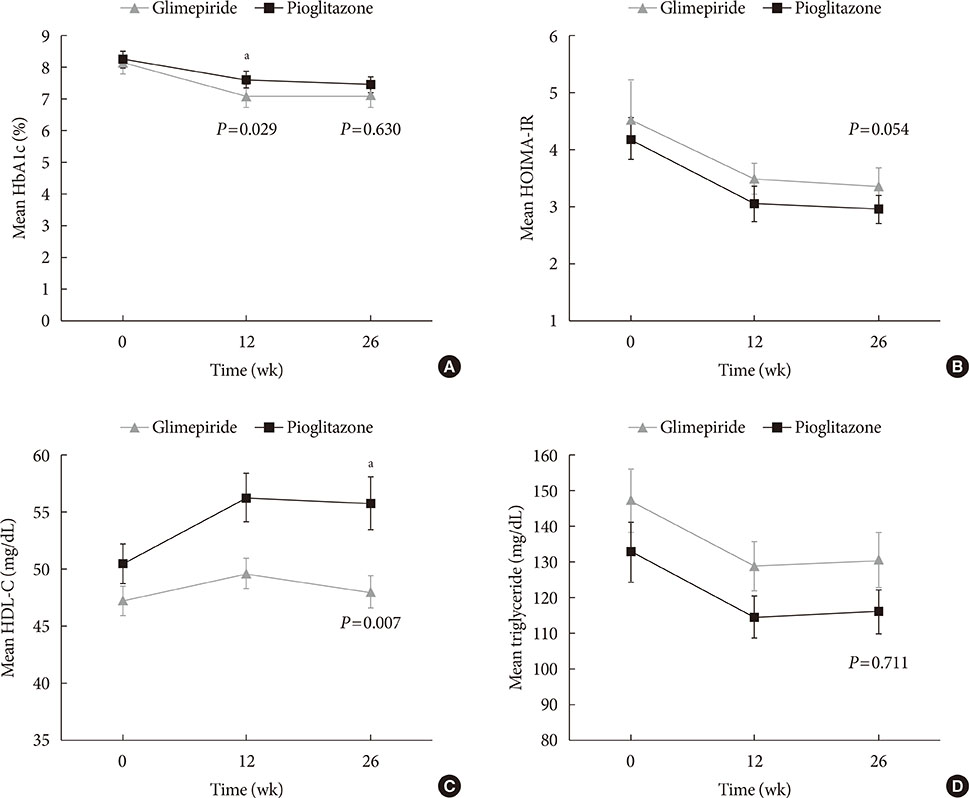
Glycosylated hemoglobin levels exhibited similar significant decreases in both groups during the treatment period (pioglitazone: −0.81%, P<0.001; glimepiride: −1.05%, P<0.001). However, the pioglitazone-treated group exhibited significantly higher high density lipoprotein cholesterol levels (P<0.001) and significantly lower homeostatic model assessment of insulin resistance values (P<0.001). Relative to pioglitazone, adding glimepiride to metformin plus alogliptin markedly increased the risk of hypoglycemia (pioglitazone: 1/69 cases [1.45%], glimepiride: 14/66 cases [21.21%]; P<0.001).
CONCLUSION
Among patients with T2DM inadequately controlled using metformin plus alogliptin, the addition of pioglitazone provided comparable glycemic control and various benefits (improvements in lipid profiles, insulin resistance, and hypoglycemia risk) relative to the addition of glimepiride.
Keyword
MeSH Terms
-
Cholesterol, HDL
Diabetes Mellitus, Type 2
Dipeptidyl-Peptidase IV Inhibitors
Drug Therapy, Combination
Hemoglobin A, Glycosylated
Humans
Hypoglycemia
Insulin Resistance
Metformin*
Sulfonylurea Compounds
Thiazolidinediones
Treatment Failure
Cholesterol, HDL
Dipeptidyl-Peptidase IV Inhibitors
Metformin
Sulfonylurea Compounds
Thiazolidinediones
Figure
Cited by 2 articles
-
Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
Ji-Yeon Park, Joonyub Lee, Yoon-Hee Choi, Kyung Wan Min, Kyung Ah Han, Kyu Jeung Ahn, Soo Lim, Young-Hyun Kim, Chul Woo Ahn, Kyung Mook Choi, Kun-Ho Yoon
Diabetes Metab J. 2024;48(5):915-928. doi: 10.4093/dmj.2023.0259.Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
Diabetes Metab J. 2024;48(5):937-948. doi: 10.4093/dmj.2023.0314.
Reference
-
1. Kim SG, Kim NH, Ku BJ, Shon HS, Kim DM, Park TS, Kim YS, Kim IJ, Choi DS. Delay of insulin initiation in patients with type 2 diabetes mellitus inadequately controlled with oral hypoglycemic agents (analysis of patient- and physician-related factors): a prospective observational DIPP-FACTOR study in Korea. J Diabetes Investig. 2017; 8:346–353.
Article2. American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41:S73–S85.3. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2018 executive summary. Endocr Pract. 2018; 24:91–120.4. Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, Lee BW, Kim HJ, Choi KM, Kim JH. Committee of Clinical Practice Guideline of Korean Diabetes Association. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association. Diabetes Metab J. 2017; 41:337–348.
Article5. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018; 41:1543–1556.
Article6. Zhang Y, McCoy RG, Mason JE, Smith SA, Shah ND, Denton BT. Second-line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care. 2014; 37:1338–1345.
Article7. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018; 41:69–78.
Article8. Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, Park JY, Song KH, Han K, Lee KU, Ko KS. Force Team for Diabetes Fact Sheet of the Korean Diabetes Association. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002–2013: nationwide population-based cohort study. Medicine (Baltimore). 2016; 95:e4018.9. Korean Diabetes Association. Diabetes fact sheet in Korea. 2018. updated 2018 May 14. Available from: http://www.diabetes.or.kr/pro/news/admin.php?category=A&code=admin&number=1546&mode=view.10. Lipska KJ, Yao X, Herrin J, McCoy RG, Ross JS, Steinman MA, Inzucchi SE, Gill TM, Krumholz HM, Shah ND. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017; 40:468–475.
Article11. Suk JH, Lee CW, Son SP, Kim MC, Ahn JH, Lee KJ, Park JY, Shin SH, Kwon MJ, Kim SS, Kim BH, Lee SH, Park JH, Kim IJ. Relationship between Cardiovascular Disease and Brachial-Ankle Pulse Wave Velocity (baPWV) in Patients with Type 2 Diabetes (REBOUND) Study Group. Current status of prescription in type 2 diabetic patients from general hospitals in Busan. Diabetes Metab J. 2014; 38:230–239.
Article12. Yabe D, Seino Y. Type 2 diabetes via β-cell dysfunction in east Asian people. Lancet Diabetes Endocrinol. 2016; 4:2–3.
Article13. Ha KH, Park CY, Jeong IK, Kim HJ, Kim SY, Kim WJ, Yoon JS, Kim IJ, Kim DJ, Kim S. Clinical characteristics of people with newly diagnosed type 2 diabetes between 2015 and 2016: difference by age and body mass index. Diabetes Metab J. 2018; 42:137–146.
Article14. Kim JD, Lee WY. Insulin secretory capacity and insulin resistance in Korean type 2 diabetes mellitus patients. Endocrinol Metab (Seoul). 2016; 31:354–360.
Article15. Vaccaro O, Masulli M, Nicolucci A, Bonora E, Del Prato S, Maggioni AP, Rivellese AA, Squatrito S, Giorda CB, Sesti G, Mocarelli P, Lucisano G, Sacco M, Signorini S, Cappellini F, Perriello G, Babini AC, Lapolla A, Gregori G, Giordano C, Corsi L, Buzzetti R, Clemente G, Di Cianni G, Iannarelli R, Cordera R, La Macchia O, Zamboni C, Scaranna C, Boemi M, Iovine C, Lauro D, Leotta S, Dall'Aglio E, Cannarsa E, Tonutti L, Pugliese G, Bossi AC, Anichini R, Dotta F, Di Benedetto A, Citro G, Antenucci D, Ricci L, Giorgino F, Santini C, Gnasso A, De Cosmo S, Zavaroni D, Vedovato M, Consoli A, Calabrese M, di Bartolo P, Fornengo P, Riccardi G. Thiazolidinediones Or Sulfonylureas Cardiovascular Accidents Intervention Trial (TOSCA.IT) study group. Italian Diabetes Society. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017; 5:887–897.16. DeFronzo RA. Pathogenesis of type 2 diabetes; metabolic and molecular implication for identifying diabetes genes. Diabetes Rev. 1997; 5:177–269.17. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004; 88:787–835.
Article18. Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996; 81:4059–4067.
Article19. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995; 333:541–549.20. Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Rev. 1998; 6:89–131.21. Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002; 34:217–224.
Article22. Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004; 89:463–478.
Article23. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004; 351:1106–1118.
Article24. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
Article25. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326.
Article26. Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, Wong E, Busse JW, Ebrahim S, Malaga G, Rios LP, Wang Y, Chen Q, Guyatt GH, Sun X. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ. 2016; 352:i610.
Article27. Kawalec P, Mikrut A, Lopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014; 30:269–283.
Article28. Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001; 24:710–719.
Article29. Leonard CE, Han X, Brensinger CM, Bilker WB, Cardillo S, Flory JH, Hennessy S. Comparative risk of serious hypoglycemia with oral antidiabetic monotherapy: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2018; 27:9–18.
Article30. DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1615–1622.
Article31. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007; 292:E871–E883.32. Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD. ACT NOW Study. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes. 2013; 62:3920–3926.
Article33. Kintscher U, Law RE. PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005; 288:E287–E291.34. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016; 165:305–315.35. Filipova E, Uzunova K, Kalinov K, Vekov T. Effects of pioglitazone therapy on blood parameters, weight and BMI: a meta-analysis. Diabetol Metab Syndr. 2017; 9:90.
Article36. Boyle PJ, King AB, Olansky L, Marchetti A, Lau H, Magar R, Martin J. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther. 2002; 24:378–396.
Article37. Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005; 11:861–866.38. Seki G, Endo Y, Suzuki M, Yamada H, Horita S, Fujita T. Role of renal proximal tubule transport in thiazolidinedione-induced volume expansion. World J Nephrol. 2012; 1:146–150.
Article39. Strowig SM, Aviles-Santa ML, Raskin P. Improved glycemic control without weight gain using triple therapy in type 2 diabetes. Diabetes Care. 2004; 27:1577–1583.
Article40. Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, Gorman M, Kelly MA, Lovejoy AM, Kernan WN. IRIS Trial Investigators. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017; 102:914–922.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study
- Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
- Comparison of Vildagliptin-Metformin and Glimepiride-Metformin Treatments in Type 2 Diabetic Patients
- Comparison of the Efficacy and Safety of Glimepiride/Metformin Fixed Combination Versus Free Combination in Patients with Type 2 Diabetes: Multicenter, Randomized, Controlled Trial
- Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial