Diabetes Metab J.
2024 Sep;48(5):937-948. 10.4093/dmj.2023.0314.
Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea
- 3Division of Endocrinology, Department of Internal Medicine, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Daegu Fatima Hospital, Daegu, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
- 7Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2559003
- DOI: http://doi.org/10.4093/dmj.2023.0314
Abstract
- Background
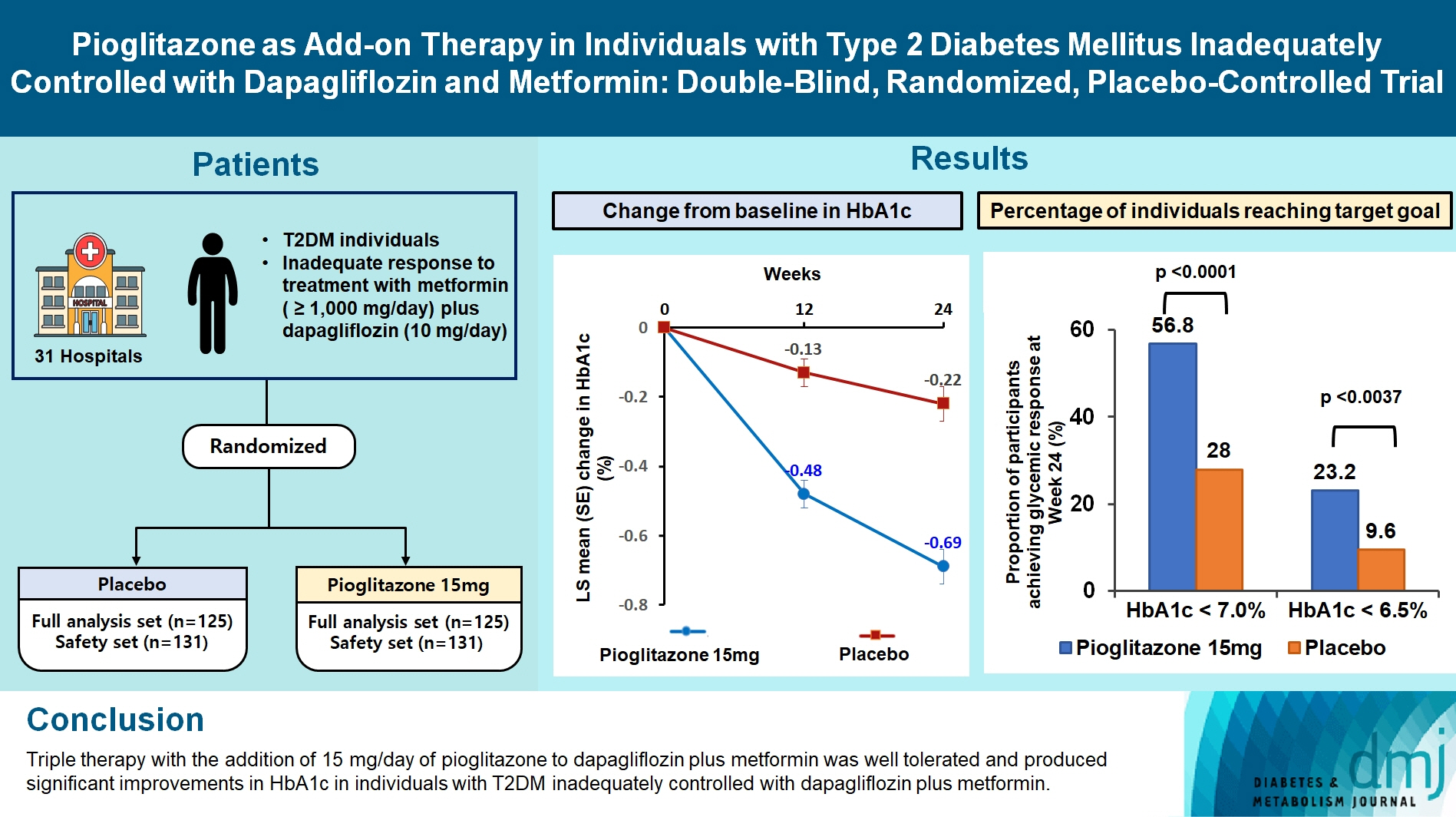
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
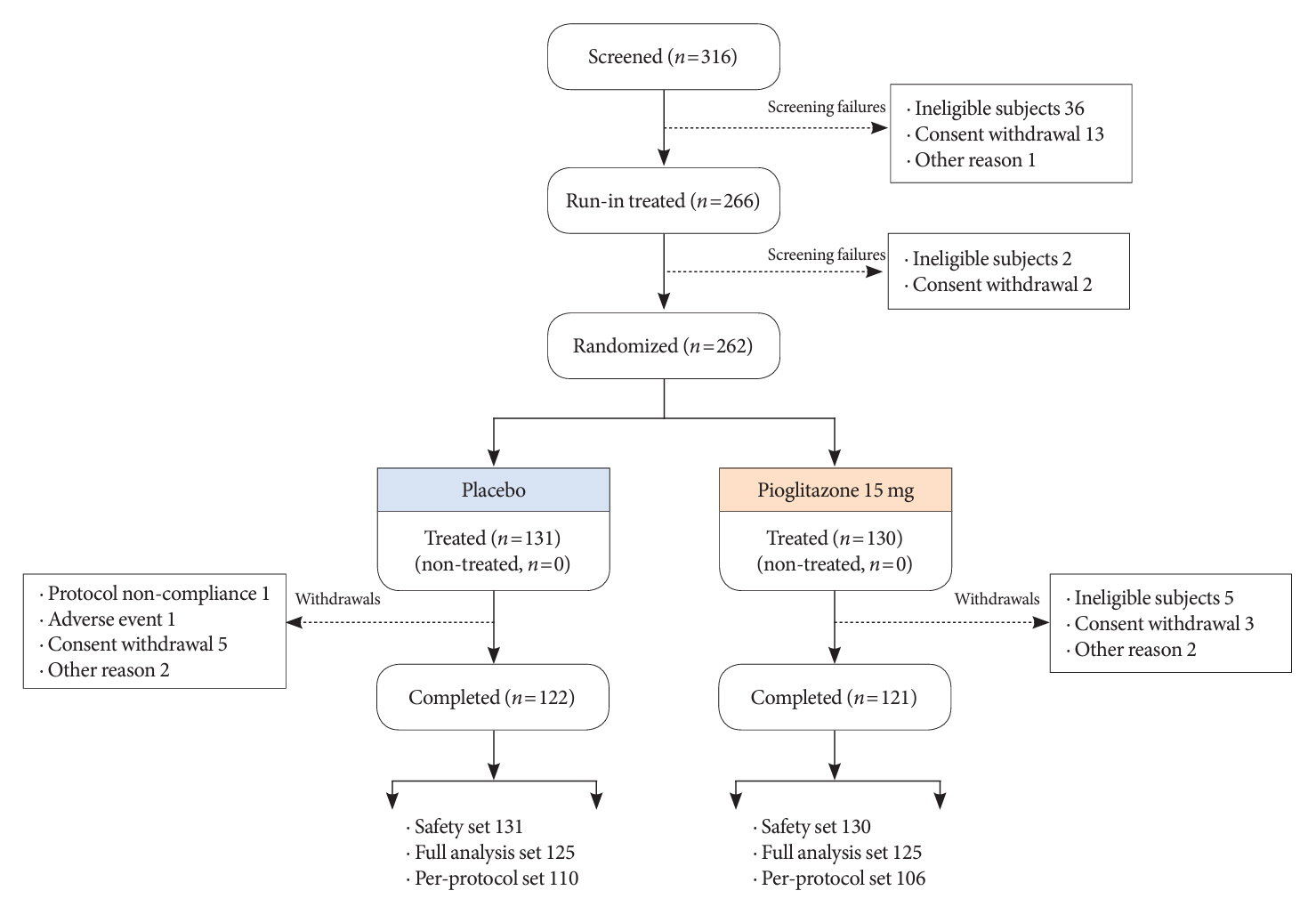
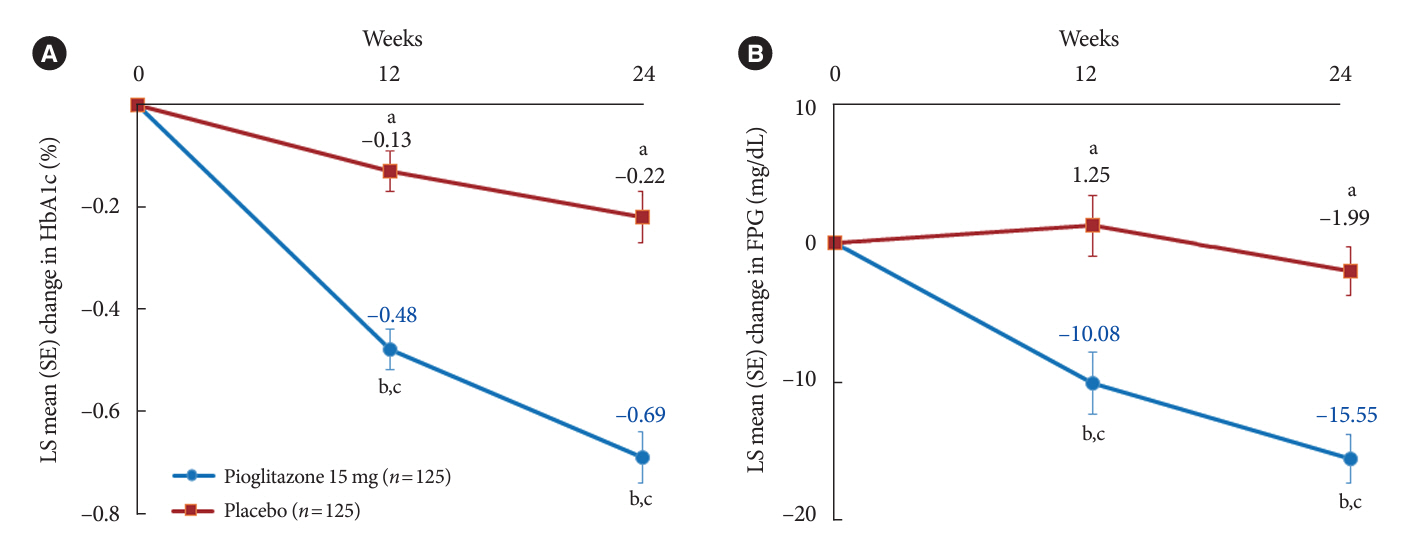
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
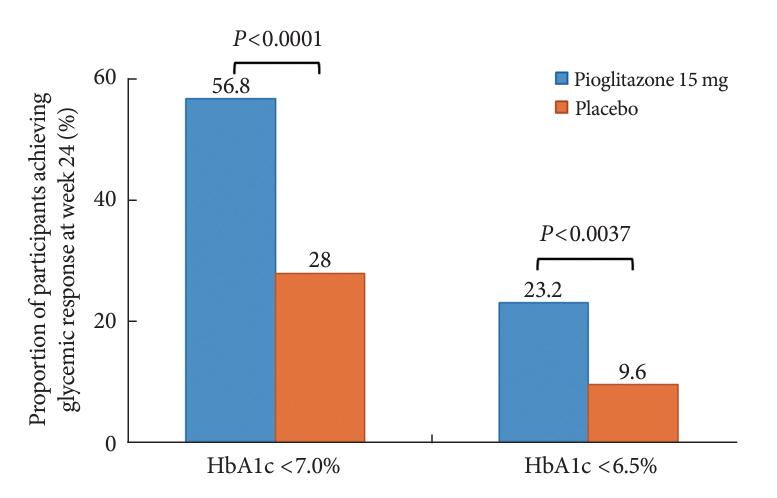
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
Keyword
Figure
Cited by 1 articles
-
Ideal Combination of Oral Hypoglycemic Agents for Patients with Type 2 Diabetes Mellitus
Hye Soon Kim
Diabetes Metab J. 2024;48(5):882-884. doi: 10.4093/dmj.2024.0479.
Reference
-
1. Kim JM, Kim SS, Kim JH, Kim MK, Kim TN, Lee SH, et al. Efficacy and safety of pioglitazone versus glimepiride after metformin and alogliptin combination therapy: a randomized, open-label, multicenter, parallel-controlled study. Diabetes Metab J. 2020; 44:67–77.
Article2. Bae J, Huh JH, Lee M, Lee YH, Lee BW. Glycaemic control with add-on thiazolidinedione or a sodium-glucose co-transporter-2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: a 24-week, randomized controlled trial. Diabetes Obes Metab. 2021; 23:609–18.
Article3. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–95.4. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S140–57.5. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022; 45:2753–86.
Article6. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005; 366:1279–89.
Article7. Viscoli CM, Kernan WN, Young LH. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 375:704.
Article8. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004; 27:256–63.
Article9. van Baar MJ, van Ruiten CC, Muskiet MH, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018; 41:1543–56.
Article10. Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care. 2007; 30:1747–52.
Article11. Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015; 38:2009–17.
Article12. Tinahones FJ, Gallwitz B, Nordaby M, Gotz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab. 2017; 19:266–74.
Article13. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.
Article14. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013; 368:1613–24.
Article15. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S158–90.16. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004; 351:1106–18.
Article17. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014; 8:1335–80.
Article18. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012; 35:1473–8.
Article19. Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014; 16:147–58.
Article20. Matthaei S, Catrinoiu D, Celinski A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015; 38:2018–24.
Article21. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 374:1321–31.
Article22. Charpentier G, Halimi S; F-PIO-100 Study Investigators. Earlier triple therapy with pioglitazone in patients with type 2 diabetes. Diabetes Obes Metab. 2009; 11:844–54.
Article23. Yang G, Li L, Tang Y, Boden G. Short-term pioglitazone treatment prevents free fatty acid-induced hepatic insulin resistance in normal rats: possible role of the resistin and adiponectin. Biochem Biophys Res Commun. 2006; 339:1190–6.
Article24. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–9.
Article25. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.26. Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, et al. Efficacy and safety of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction according to age: the DELIVER Trial. Circ Heart Fail. 2022; 15:e010080.
Article27. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–24.28. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003; 108:2941–8.29. Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring). 2009; 17:1017–22.30. Iketani R, Imai S. Prescription trends for the antidiabetic agents used to treat type 2 diabetes mellitus in Japan from 2012-2020: a time-series analysis. Biol Pharm Bull. 2023; 46:592–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension