Endocrinol Metab.
2023 Jun;38(3):328-337. 10.3803/EnM.2023.1688.
Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Endocrinology and Metabolism, Nowon Eulji Medical Center, Eulji University, Seoul, Korea
- 3Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, College of Medicine, Hallym University, Hwaseong, Korea
- 4Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 5Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 6Department of Endocrinology and Metabolism, Kyung Hee University Hospital, College of Medicine, Kyung Hee University, Seoul, Korea
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 9Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 10Department of Internal Medicine, Chosun University Hospital, Chosun University College of Medicine, Gwangju, Korea
- 11Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 12Division of Endocrinology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- KMID: 2543319
- DOI: http://doi.org/10.3803/EnM.2023.1688
Abstract
- Background
This study evaluated the efficacy and safety of add-on gemigliptin in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycemic control with metformin and dapagliflozin.
Methods
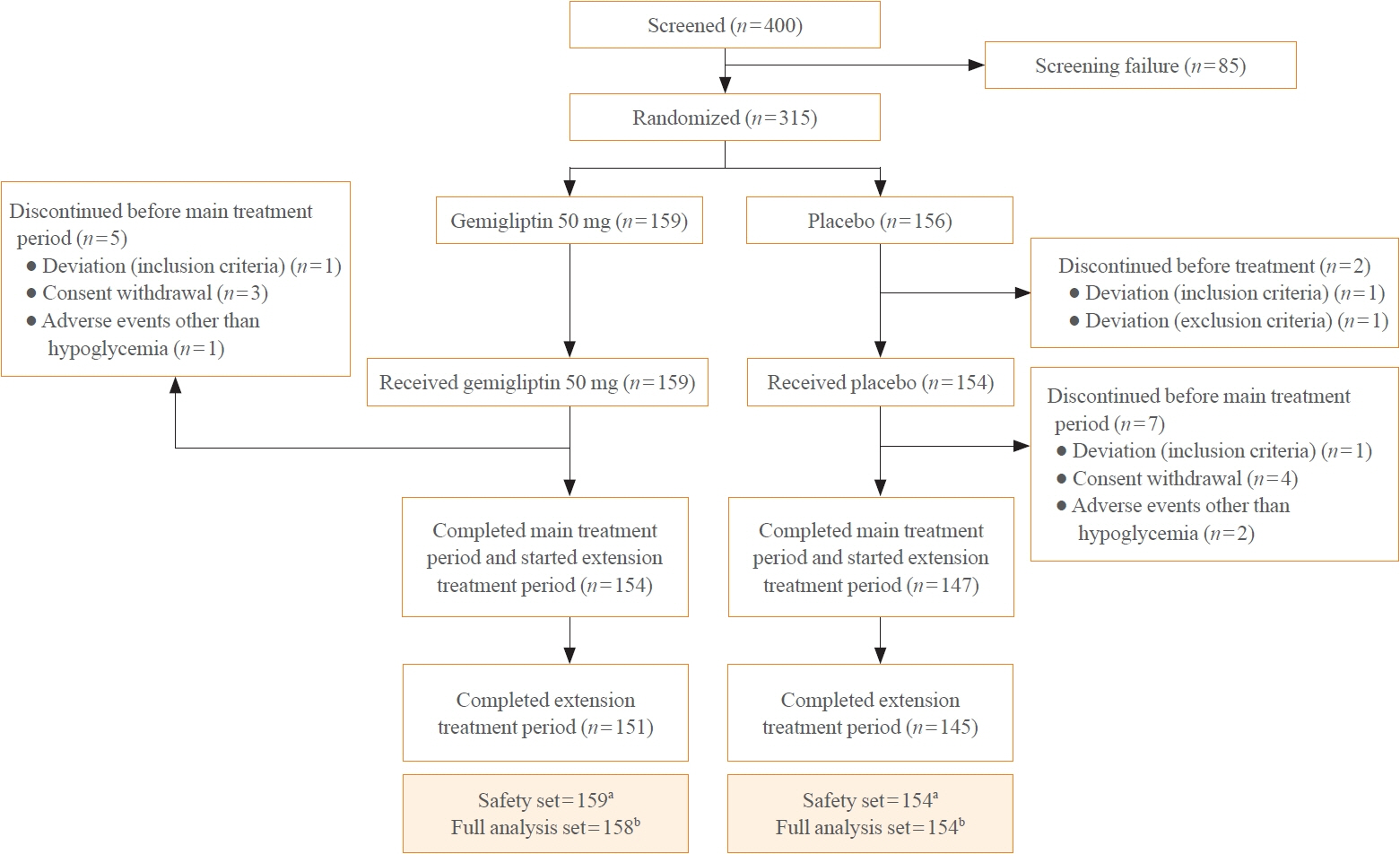
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study, 315 patients were randomized to receive either gemigliptin 50 mg (n=159) or placebo (n=156) with metformin and dapagliflozin for 24 weeks. After the 24-week treatment, patients who received the placebo were switched to gemigliptin, and all patients were treated with gemigliptin for an additional 28 weeks.
Results
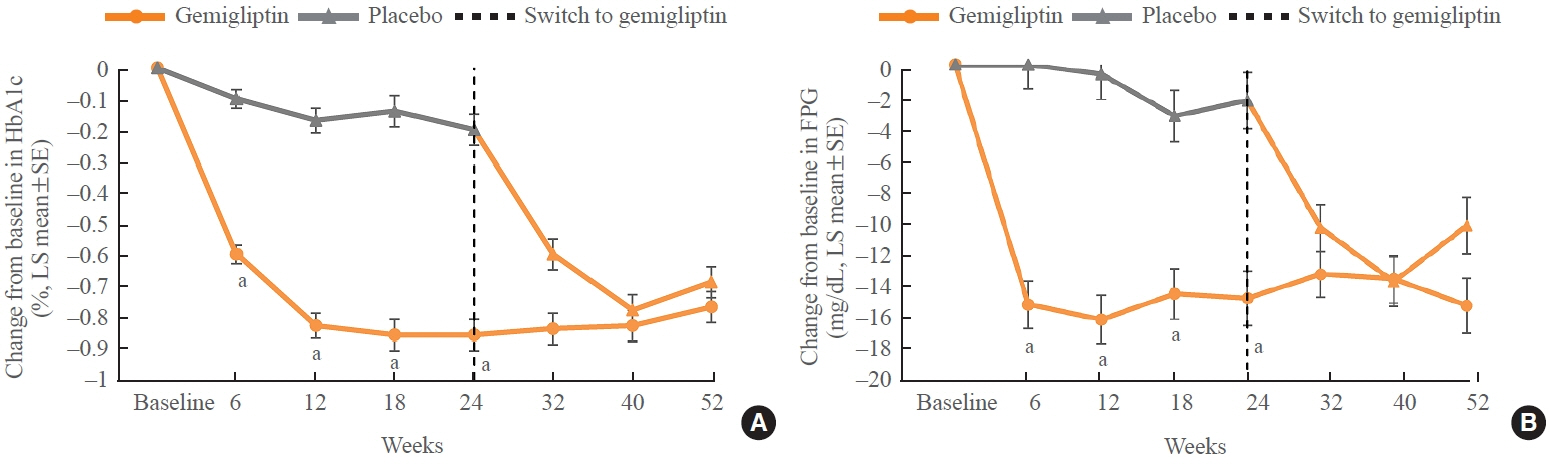
The baseline characteristics were similar between the two groups, except for body mass index. At week 24, the least squares mean difference (standard error) in hemoglobin A1c (HbA1c) changes was –0.66% (0.07) with a 95% confidence interval of –0.80% to –0.52%, demonstrating superior HbA1c reduction in the gemigliptin group. After week 24, the HbA1c level significantly decreased in the placebo group as gemigliptin was administered, whereas the efficacy of HbA1c reduction was maintained up to week 52 in the gemigliptin group. The safety profiles were similar: the incidence rates of treatment-emergent adverse events up to week 24 were 27.67% and 29.22% in the gemigliptin and placebo groups, respectively. The safety profiles after week 24 were similar to those up to week 24 in both groups, and no new safety findings, including hypoglycemia, were noted.
Conclusion
Add-on gemigliptin was well tolerated, providing comparable safety profiles and superior efficacy in glycemic control over placebo for long-term use in patients with T2DM who had poor glycemic control with metformin and dapagliflozin.
Keyword
Figure
Reference
-
1. DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014; 27:100–12.
Article2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007; 298:194–206.
Article3. Holst JJ, Deacon CF. Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol. 2004; 4:589–96.
Article4. Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008; 4:753–68.5. Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998; 47:1663–70.
Article6. Korean Diabetes Association. Clinical practice guidelines for diabetes. 7th ed. Seoul: Korean Diabetes Association;2021.7. Shah NK, Deeb WE, Choksi R, Epstein BJ. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2012; 32:80–94.
Article8. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010; 375:2223–33.
Article9. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:323–34.
Article10. Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:613–21.
Article11. Dey J. SGLT2 inhibitor/DPP-4 inhibitor combination therapy: complementary mechanisms of action for management of type 2 diabetes mellitus. Postgrad Med. 2017; 129:409–20.12. Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016; 12:1407–17.
Article13. Matthaei S, Catrinoiu D, Celinski A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015; 38:2018–24.
Article14. Matthaei S, Aggarwal N, Garcia-Hernandez P, Iqbal N, Chen H, Johnsson E, et al. One-year efficacy and safety of saxagliptin add-on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016; 18:1128–33.
Article15. Tinahones FJ, Gallwitz B, Nordaby M, Gotz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab. 2017; 19:266–74.
Article16. Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:410–6.
Article17. Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013; 15:523–30.
Article18. Lim S, Han KA, Yu J, Chamnan P, Kim ES, Yoon KH, et al. Efficacy and safety of initial combination therapy with gemigliptin and metformin compared with monotherapy with either drug in patients with type 2 diabetes: a double-blind randomized controlled trial (INICOM study). Diabetes Obes Metab. 2017; 19:87–97.
Article19. Ahn CH, Han KA, Yu JM, Nam JY, Ahn KJ, Oh TK, et al. Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study). Diabetes Obes Metab. 2017; 19:635–43.
Article20. Yoon SA, Han BG, Kim SG, Han SY, Jo YI, Jeong KH, et al. Efficacy, safety and albuminuria-reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: a 12-week, double-blind randomized study (the GUARD Study). Diabetes Obes Metab. 2017; 19:590–8.
Article21. Cho YM, Deerochanawong C, Seekaew S, Suraamornkul S, Benjachareonwong S, Sattanon S, et al. Efficacy and safety of gemigliptin as add-on therapy to insulin, with or without metformin, in patients with type 2 diabetes mellitus (ZEUS II study). Diabetes Obes Metab. 2020; 22:123–7.
Article22. Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009; 25:2401–11.23. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017; 40:155–7.24. American Diabetes Association. Standards of medical care in diabetes: 2022. Diabetes Care. 2022; 45(Suppl 1):S1–264.25. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, Hansen L, Chen H, Johnsson E, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016; 18:1134–7.
Article26. Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab. 2011; 13:1088–96.
Article27. Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013; 36:2508–15.28. AstraZeneca Pharmaceutical. Farxiga [Internet]. Silver Spring: U.S. Food and Drug Administration;2022. [cited 2023 May 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/202293s028lbl.pdf.29. Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018; 20:740–4.
Article30. Li D, Shi W, Wang T, Tang H. SGLT2 inhibitor plus DPP-4 inhibitor as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018; 20:1972–6.
Article31. EMA Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet]. London: European Medicines Agency;2012. [cited 2023 May 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf.32. National Institute of Food and Drug Safety Evaluation. Guideline on clinical trials of oral hypoglycemic agent [Internet]. Cheongju: Ministry of Food and Drug Safety;2015. [cited 2023 May 12]. Available from: https://www.mfds.go.kr/index.do.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
- A Review on Efficacy and Safety of SGLT2 Inhibitors as Add-on Therapy with Metformin
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)