Diabetes Metab J.
2023 Nov;47(6):796-807. 10.4093/dmj.2022.0315.
Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Bundang Jesaeng Hospital, Seongnam, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 9Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- 10Clinical Development Center, Daewoong Pharmaceutical Co. Ltd., Seoul, Korea
- 11Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2548156
- DOI: http://doi.org/10.4093/dmj.2022.0315
Abstract
- Background
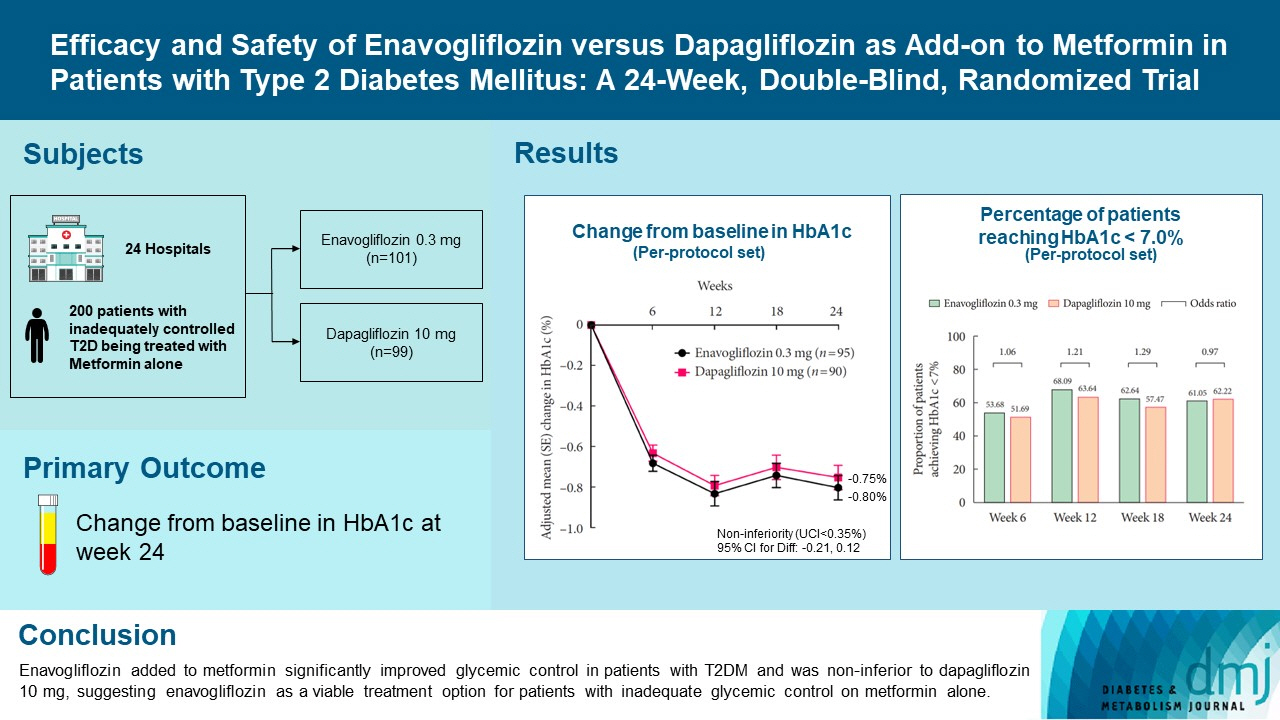
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
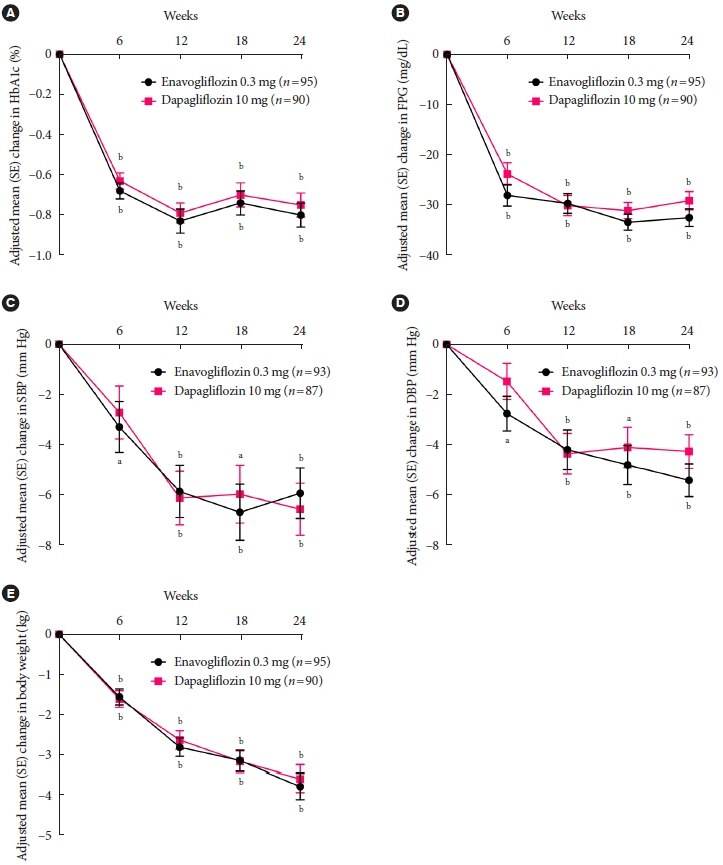
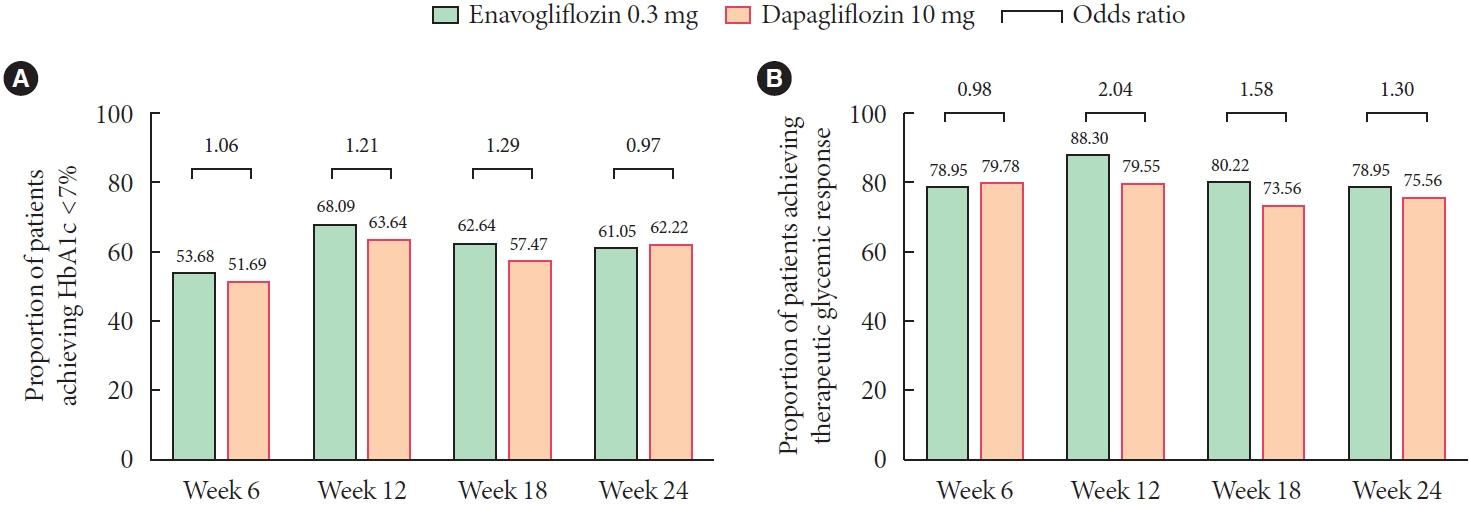
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone.
Keyword
Figure
Cited by 2 articles
-
Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes Metab J. 2023;47(6):769-770. doi: 10.4093/dmj.2023.0351.Study Design and Protocol for a Randomized Controlled Trial of Enavogliflozin to Evaluate Cardiorenal Outcomes in Type 2 Diabetes (ENVELOP)
Nam Hoon Kim, Soo Lim, In-Kyung Jeong, Eun-Jung Rhee, Jun Sung Moon, Ohk-Hyun Ryu, Hyuk-Sang Kwon, Jong Chul Won, Sang Soo Kim, Sang Yong Kim, Bon Jeong Ku, Heung Yong Jin, Sin Gon Kim, Bong-Soo Cha
Diabetes Metab J. 2025;49(2):225-234. doi: 10.4093/dmj.2024.0238.
Reference
-
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157:107843.
Article2. Neumiller JJ, Umpierrez GE. 2018 Standards of care update: pharmacologic approaches to glycemic management in people with type 2 diabetes. Diabetes Spectr. 2018; 31:254–60.
Article3. Dash RP, Babu RJ, Srinivas NR. Comparative pharmacokinetics of three SGLT-2 inhibitors sergliflozin, remogliflozin and ertugliflozin: an overview. Xenobiotica. 2017; 47:1015–26.
Article4. Garcia-Ropero A, Badimon JJ, Santos-Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol. 2018; 14:1287–302.
Article5. Sheu WH, Chan SP, Matawaran BJ, Deerochanawong C, Mithal A, Chan J, et al. Use of SGLT-2 inhibitors in patients with type 2 diabetes mellitus and abdominal obesity: an Asian perspective and expert recommendations. Diabetes Metab J. 2020; 44:11–32.
Article6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.
Article7. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–57.
Article8. Cai X, Yang W, Gao X, Chen Y, Zhou L, Zhang S, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring). 2018; 26:70–80.
Article9. Gill HK, Kaur P, Mahendru S, Mithal A. Adverse effect profile and effectiveness of sodium glucose co-transporter 2 inhibitors (SGLT2i): a prospective real-world setting study. Indian J Endocrinol Metab. 2019; 23:50–5.10. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–57.
Article11. Choi MK, Nam SJ, Ji HY, Park MJ, Choi JS, Song IS. Comparative pharmacokinetics and pharmacodynamics of a novel sodium-glucose cotransporter 2 inhibitor, DWP16001, with dapagliflozin and ipragliflozin. Pharmaceutics. 2020; 12:268.
Article12. Kim JH, Kim DK, Choi WG, Ji HY, Choi JS, Song IS, et al. In vitro metabolism of DWP16001, a novel sodium-glucose cotransporter 2 inhibitor, in human and animal hepatocytes. Pharmaceutics. 2020; 12:865.
Article13. Hwang JG, Lee S, Huh W, Han J, Oh J, Jang IJ, et al. Dose-dependent glucosuria of DWP16001, a novel selective sodiumglucose cotransporter-2 inhibitor, in healthy subjects. Br J Clin Pharmacol. 2022; 88:4100–10.
Article14. Yang YS, Min KW, Park SO, Kim KS, Yu JM, Hong EG, et al. Efficacy and safety of monotherapy with enavogliflozin in Korean patients with type 2 diabetes mellitus: results of a 14 week, multi-center, randomized, double-blind, placebo-controlled, phase 2 trial. 2020 International Congress of Diabetes and Metabolism;. 2020 Sep 18-19; Online. Available from: https:// icdm2020.diabetes.or.kr/program/oral_list.php.15. Food and Drug Administration: Draft guidance for industry on diabetes mellitus. Developing drugs and therapeutic biologics for treatment and prevention: guidance document. Available from: https://www.regulations.gov/document/FDA-2008-D-0118-0003 (cited 2023 Feb 1).16. European Medicines Agency: Clinical investigation of medicinal products in the treatment or prevention diabetes mellitus. Available from: https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus (cited 2023 Feb 1).17. Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011; 34:2015–22.18. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012; 66:446–56.
Article19. Dharmalingam M, Aravind SR, Thacker H, Paramesh S, Mohan B, Chawla M, et al. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs. 2020; 80:587–600.
Article20. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000 [cited 2023 Feb 1]. Available from: https://apps.who.int/iris/handle/10665/206936.21. Yang W, Han P, Min KW, Wang B, Mansfield T, T’Joen C, et al. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: a randomized controlled trial. J Diabetes. 2016; 8:796–808.
Article22. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010; 375:2223–33.
Article23. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010; 33:2217–24.24. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011; 13:928–38.
Article25. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012; 35:1473–8.
Article26. Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014; 53:17–27.
Article27. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–79.
Article28. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014; 124:509–14.
Article29. Kaneto H, Obata A, Kimura T, Shimoda M, Kinoshita T, Matsuoka TA, et al. Unexpected pleiotropic effects of SGLT2 inhibitors: pearls and pitfalls of this novel antidiabetic class. Int J Mol Sci. 2021; 22:3062.
Article30. Op den Kamp YJ, Gemmink A, de Ligt M, Dautzenberg B, Kornips E, Jorgensen JA, et al. Effects of SGLT2 inhibitor dapagliflozin in patients with type 2 diabetes on skeletal muscle cellular metabolism. Mol Metab. 2022; 66:101620.
Article31. Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009; 182(6 Suppl):S51–6.
Article32. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003; 26:510–3.
Article33. Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014; 103:373–81.
Article34. Nauck MA, Del Prato S, Duran-Garcia S, Rohwedder K, Langkilde AM, Sugg J, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. 2014; 16:1111–20.35. Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015; 32:531–41.
Article36. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012; 156:405–15.
Article37. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, doubleblind, placebo-controlled 102-week trial. BMC Med. 2013; 11:43.
Article38. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014; 8:1335–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Study Design and Protocol for a Randomized Controlled Trial of Enavogliflozin to Evaluate Cardiorenal Outcomes in Type 2 Diabetes (ENVELOP)
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
- Response: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes metab J 2019;43;276-86)